Phentermine
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Adipex-p, Duromine, Metermine, Suprenza |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682187 |
| License data |
|
| Pregnancy category |
|
| Dependence liability |
Low |
| Addiction liability |
Very low |
| Routes of administration |
by mouth |
| ATC code | A08AA01 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | High (almost complete) |
| Protein binding | Approximately 96.3% |
| Metabolism | Hepatic |
| Biological half-life | 25 hours, urinary pH-dependent |
| Excretion | Urinary (62–85% unchanged) |
| Identifiers | |
|
|
| Synonyms | α-methyl-amphetamine α,α-dimethylphenethylamine |
| CAS Number |
122-09-8 |
| PubChem (CID) | 4771 |
| IUPHAR/BPS | 7269 |
| DrugBank |
DB00191 |
| ChemSpider |
4607 |
| UNII |
C045TQL4WP |
| KEGG |
D05458 |
| ChEBI |
CHEBI:8080 |
| ChEMBL |
CHEMBL1574 |
| ECHA InfoCard | 100.004.112 |
| Chemical and physical data | |
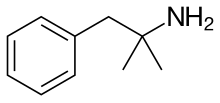
| Formula | C10H15N |
| Molar mass | 149.233 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
Phentermine (α,α-Dimethylphenethylamine), a contraction of "phenyl-tertiary-butylamine", is a psychostimulant drug of the substituted amphetamine chemical class, with pharmacology similar to amphetamine. It is used medically as an appetite suppressant for short term use, as an adjunct to exercise and reducing calorie intake.
It has cardiovascular, gastrointestinal, and CNS side effects; rare cases of pulmonary hypertension and cardiac valvular disease have been reported. It should not be used by people who have a history of drug abuse, have any cardiovascular disease, hyperthyroidism, glaucoma, peptic ulcers, prostatic hypertrophy, or epilepsy, or are pregnant, planning to become pregnant, or breast-feeding. It should not be taken by anyone taking a monoamine oxidase inhibitor, and people should not drink alcohol while they are taking it.
It was first introduced in 1959, and became part of the drug combination Fen-phen that was withdrawn from the market in 1997 due to the fenfluramine component damaging people's heart valves. In 2012 a different combination drug, phentermine/topiramate was approved in the US.
...
Wikipedia
