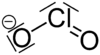
Sodium chlorite
|
|
|||
|
|
|||
 |
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
Sodium chlorite
|
|||
| Other names
Chlorous acid, sodium salt
Textone |
|||
| Identifiers | |||
|
7758-19-2 49658-21-1 (trihydrate) |
|||
| 3D model (Jmol) | Interactive image | ||
| ChEBI |
CHEBI:78667 |
||
| ChemSpider |
22860 |
||
| ECHA InfoCard | 100.028.942 | ||
| EC Number | 231-836-6 | ||
| KEGG |
C19523 |
||
| PubChem | 23668197 | ||
| RTECS number | VZ4800000 | ||
| UNII |
G538EBV4VF |
||
| UN number | 1496 | ||
|
|||
|
|||
| Properties | |||
| NaClO2 | |||
| Molar mass | 90.442 g/mol (anhydrous) 144.487 g/mol (trihydrate) |
||
| Appearance | white solid | ||
| Odor | odorless | ||
| Density | 2.468 g/cm3, solid | ||
| Melting point | anhydrous decomposes at 180–200 °C trihydrate decomposes at 38 °C |
||
| 75.8 g/100 mL (25 °C) 122 g/100 mL (60 °C) |
|||
| Solubility | slightly soluble in methanol, ethanol | ||
| Acidity (pKa) | 10-11 | ||
| Structure | |||
| monoclinic | |||
| Thermochemistry | |||
|
Std enthalpy of
formation (ΔfH |
-307.0 kJ/mol | ||
| Pharmacology | |||
| D03AX11 (WHO) | |||
| Hazards | |||
| Safety data sheet | ICSC 1045 | ||
| NFPA 704 | |||
| Flash point | Non-flammable | ||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
350 mg/kg (rat, oral) | ||
| Related compounds | |||
|
Other anions
|
Sodium chloride Sodium hypochlorite Sodium chlorate Sodium perchlorate |
||
|
Other cations
|
Potassium chlorite Barium chlorite |
||
|
Related compounds
|
Chlorine dioxide Chlorous acid |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Sodium chlorite (NaClO2) is a chemical compound used in the manufacturing of paper and as a disinfectant.
The main application of sodium chlorite is the generation of chlorine dioxide for bleaching and stripping of textiles, pulp, and paper. It is also used for disinfection of municipal water treatment plants after conversion to chlorine dioxide. An advantage in this application, as compared to the more commonly used chlorine, is that trihalomethanes (such as chloroform) are not produced from organic contaminants. Chlorine dioxide generated from sodium chlorite is approved by FDA under some conditions for disinfecting water used to wash fruits, vegetables, and poultry.
Sodium chlorite, NaClO2, sometimes in combination with zinc chloride, also finds application as a component in therapeutic rinses, mouthwashes, toothpastes and gels, mouth sprays, as preservative in eye drops, and in contact lens cleaning solution under the trade name Purite.
Under the brand name Oxine it is used for sanitizing air ducts and HVAC/R systems and animal containment areas (walls, floors, and other surfaces).
Neuraltus Pharmaceuticals is investigating a drug that they refer to as NP001 for treatment of amyotrophic lateral sclerosis. Some people with ALS have concluded that NP001 is a formulation of sodium chlorite, and are ordering the chemical and self-dosing outside of any scientific study. Preliminary results suggest that this sodium chlorite treatment is less effective than NP001. One seller of “Miracle Mineral Solution” (a mixture of sodium chlorite and water also known as "MMS") was recently convicted in the United States for marketing a toxic chemical as a miracle cure, as sodium chlorite cannot be legally sold for human consumption in the country.
In organic synthesis, sodium chlorite is frequently used as a reagent in the Pinnick oxidation for the oxidation of aldehydes to carboxylic acids. The reaction is usually performed in monosodium phosphate buffered solution in the presence of a chlorine scavenger (usually 2-methyl-2-butene).
...
Wikipedia