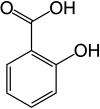
Salicylate
|
|
|||
 |
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
2-Hydroxybenzoic acid
|
|||
| Identifiers | |||
|
69-72-7 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChEBI |
CHEBI:16914 |
||
| ChEMBL |
ChEMBL424 |
||
| ChemSpider |
331 |
||
| DrugBank |
DB00936 |
||
| ECHA InfoCard | 100.000.648 | ||
| EC Number | 200-712-3 | ||
| 4306 | |||
| KEGG |
D00097 |
||
| PubChem | 338 | ||
| RTECS number | VO0525000 | ||
| UNII |
O414PZ4LPZ |
||
|
|||
|
|||
| Properties | |||
| C7H6O3 | |||
| Molar mass | 138.12 g·mol−1 | ||
| Appearance | colorless to white crystals | ||
| Odor | odorless | ||
| Density | 1.443 g/cm3 (20 °C) | ||
| Melting point | 158.6 °C (317.5 °F; 431.8 K) | ||
| Boiling point | 200 °C (392 °F; 473 K) decomposes 211 °C (412 °F; 484 K) at 20 mmHg |
||
| sublimes at 76 °C | |||
| 1.24 g/L (0 °C) 2.48 g/L (25 °C) 4.14 g/L (40 °C) 17.41 g/L (75 °C) 77.79 g/L (100 °C) |
|||
| Solubility | soluble in ether, CCl4, benzene, propanol, acetone, ethanol, oil of turpentine, toluene | ||
| Solubility in benzene | 0.46 g/100 g (11.7 °C) 0.775 g/100 g (25 °C) 0.991 g/100 g (30.5 °C) 2.38 g/100 g (49.4 °C) 4.4 g/100 g (64.2 °C) |
||
| Solubility in chloroform | 2.22 g/100 mL (25 °C) 2.31 g/100 mL (30.5 °C) |
||
| Solubility in methanol | 40.67 g/100 g (−3 °C) 62.48 g/100 g (21 °C) |
||
| Solubility in olive oil | 2.43 g/100 g (23 °C) | ||
| Solubility in acetone | 39.6 g/100 g (23 °C) | ||
| log P | 2.26 | ||
| Vapor pressure | 10.93 mPa | ||
| Acidity (pKa) | 1 = 2.97 (25 °C) 2 = 13.82 (20 °C) |
||
| UV-vis (λmax) | 210 nm, 234 nm, 303 nm (4 mg % in ethanol) | ||
| -72.23·10−6 cm3/mol | |||
|
Refractive index (nD)
|
1.565 (20 °C) | ||
| Thermochemistry | |||
|
Std enthalpy of
formation (ΔfH |
-589.9 kJ/mol | ||
|
Std enthalpy of
combustion (ΔcH |
3.025 MJ/mol | ||
| Pharmacology | |||
| A01AD05 (WHO) B01AC06 (WHO) D01AE12 (WHO) N02BA01 (WHO) S01BC08 (WHO) | |||
| Hazards | |||
| Safety data sheet | MSDS | ||
| GHS pictograms |
 
|
||
| GHS signal word | Danger | ||
| H302, H318 | |||
| P280, P305+351+338 | |||
|
EU classification (DSD)
|
|||
| R-phrases | R22, R38, R41, R61 | ||
| S-phrases | S22, S26, S36, S37, S39 | ||
| Eye hazard | Severe irritation | ||
| Skin hazard | Mild irritation | ||
| NFPA 704 | |||
| Flash point | 157 °C (315 °F; 430 K) closed cup |
||
| 540 °C (1,004 °F; 813 K) | |||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
480 mg/kg (mice, oral) | ||
| Related compounds | |||
|
Related compounds
|
Methyl salicylate, Benzoic acid, Phenol, Aspirin, 4-Hydroxybenzoic acid, Magnesium salicylate, Choline salicylate, Bismuth subsalicylate, Sulfosalicylic acid |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Salicylic acid (from Latin salix, willow tree) is a monohydroxybenzoic acid, a type of phenolic acid, and a beta hydroxy acid. It has the formula C7H6O3. This colorless crystalline organic acid is widely used in organic synthesis and functions as a plant hormone. It is derived from the metabolism of salicin. In addition to serving as an important active metabolite of aspirin (acetylsalicylic acid), which acts in part as a prodrug to salicylic acid, it is probably best known for its use as a key ingredient in topical anti-acne products. The salts and esters of salicylic acid are known as salicylates. The medicinal part of the plant is the inner bark.
It is on the WHO Model List of Essential Medicines, the most important medications needed in a basic health system.
Salicylic acid as a medication is used most commonly to help remove the outer layer of the skin. As such it is used to treat warts, psoriasis, dandruff, acne, ringworm, and ichthyosis.
As with other hydroxy acids, salicylic acid is a key ingredient in many skin-care products for the treatment of seborrhoeic dermatitis, acne, psoriasis, calluses, corns, keratosis pilaris, acanthosis nigricans, ichthyosis and warts.
...
Wikipedia