Proton
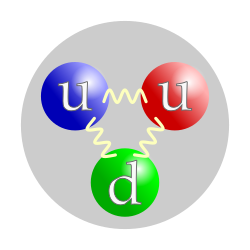
The quark structure of a proton. The color assignment of individual quarks is arbitrary, but all three colors must be present. Forces between quarks are mediated by gluons.
|
|
| Classification | Baryon |
|---|---|
| Composition | 2 up quarks, 1 down quark |
| Statistics | Fermionic |
| Interactions | Gravity, electromagnetic, weak, strong |
| Symbol |
p , p+ , N+ |
| Antiparticle | Antiproton |
| Theorized | William Prout (1815) |
| Discovered | Eugen Goldstein (1886) and Ernest Rutherford (1917–1919, named by him, 1920) |
| Mass |
1.672621898(21)×10−27 kg |
| Mean lifetime | > 2.1×1029 years (stable) |
| Electric charge |
+1 e 1.6021766208(98)×10−19 C |
| Charge radius | 0.8751(61) fm |
| Electric dipole moment | < 5.4×10−24 e⋅cm |
| Electric polarizability | 1.20(6)×10−3 fm3 |
| Magnetic moment |
1.4106067873(97)×10−26 J⋅T−1 |
| Magnetic polarizability | 1.9(5)×10−4 fm3 |
| Spin | 1/2 |
| Isospin | 1/2 |
| Parity | +1 |
| Condensed | I(JP) = 1/2(1/2+) |
1.672621898(21)×10−27 kg
938.2720813(58) MeV/c2
1.4106067873(97)×10−26 J⋅T−1
1.5210322053(46)×10−3 μB
A proton is a subatomic particle, symbol
p
or
p+
, with a positive electric charge of +1e elementary charge and mass slightly less than that of a neutron. Protons and neutrons, each with masses of approximately one atomic mass unit, are collectively referred to as "nucleons". One or more protons are present in the nucleus of every atom; they are a necessary part of the nucleus. The number of protons in the nucleus is the defining property of an element, and is referred to as the atomic number (represented by the symbol Z). Since each element has a unique number of protons, each element has its own unique atomic number. The word proton is Greek for "first", and this name was given to the hydrogen nucleus by Ernest Rutherford in 1920. In previous years, Rutherford had discovered that the hydrogen nucleus (known to be the lightest nucleus) could be extracted from the nuclei of nitrogen by atomic collisions. Protons were therefore a candidate to be a fundamental particle, and hence a building block of nitrogen and all other heavier atomic nuclei.
...
Wikipedia
