Prograf
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Prograf, Advagraf, Protopic |
| Pregnancy category |
|
| Routes of administration |
Topical, oral, iv |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 24% (5–67%), less after eating food rich in fat |
| Protein binding | ≥98.8% |
| Metabolism | Hepatic CYP3A4, CYP3A5 |
| Biological half-life | 11.3 h for transplant patients (range 3.5–40.6 h) |
| Excretion | Mostly faecal |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.155.367 |
| Chemical and physical data | |
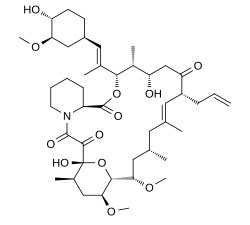
| Formula | C44H69NO12 |
| Molar mass | 804.018 g/mol |
| 3D model (JSmol) | |
|
|
|
|
|
|
|
Tacrolimus (also FK-506 or fujimycin, trade names Prograft, Advagraf, Protopic) is an immunosuppressive drug used mainly after allogeneic organ transplant to lower the risk of organ rejection. It achieves this by inhibiting the production of interleukin-2, a molecule that promotes the development and proliferation of T cells, which are vital to the body's learned (or adaptive) immune response. Tacrolimus is also used in the treatment of other T cell-mediated diseases such as eczema (for which it is applied to the skin in a medicated ointment), severe refractory uveitis after bone marrow transplants, exacerbations of minimal change disease, Kimura's disease, and the skin condition vitiligo.
Chemically it is a 23-membered macrolide lactone that was first discovered in 1987 from the fermentation broth of a Japanese soil sample that contained the bacterium Streptomyces tsukubaensis.
It has similar immunosuppressive properties to ciclosporin, but is much more potent. Immunosuppression with tacrolimus was associated with a significantly lower rate of acute rejection compared with ciclosporin-based immunosuppression (30.7% vs 46.4%) in one study. Clinical outcome is better with tacrolimus than with ciclosporin during the first year of liver transplantation. Long-term outcome has not been improved to the same extent. Tacrolimus is normally prescribed as part of a post-transplant cocktail including steroids, mycophenolate, and IL-2 receptor inhibitors such as basiliximab. Dosages are titrated to target blood levels.
...
Wikipedia
