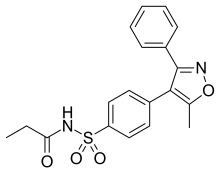
Parecoxib
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| License data | |
| Pregnancy category |
|
| Routes of administration |
Intravenous and intramuscular |
| ATC code | M01AH04 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% |
| Protein binding | 98% |
| Metabolism |
Hepatic to valdecoxib and propionic acid extensively involved (mainly CYP3A4 and 2C9) |
| Biological half-life | 22 minutes (parecoxib) 8 hours (valdecoxib) |
| Excretion | Renal (70%, metabolites) |
| Identifiers | |
|
|
| CAS Number |
202409-33-4 |
| PubChem (CID) | 119828 |
| IUPHAR/BPS | 2895 |
| DrugBank |
DB08439 |
| ChemSpider |
106990 |
| UNII |
9TUW81Y3CE |
| ChEBI |
CHEBI:73038 |
| ChEMBL |
CHEMBL1206690 |
| ECHA InfoCard | 100.230.078 |
| Chemical and physical data | |
| Formula | C19H18N2O4S |
| Molar mass | 370.422 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
|
|
|
Parecoxib is a water-soluble and injectable prodrug of valdecoxib. It is marketed as Dynastat in the European Union. Parecoxib is a COX2 selective inhibitor in the same category as celecoxib (Celebrex) and rofecoxib (Vioxx). As it is injectable, it can be used perioperatively when patients are unable to take oral medications. It is approved through much of Europe for short term perioperative pain control much in the same way ketorolac (Toradol) is used in the United States. However, unlike ketorolac, parecoxib has no effect on platelet function and therefore does not promote bleeding during or after surgery. In addition, ketorolac has a much higher gastrointestinal toxicity profile compared to most other nonsteroidal antiinflammatory drugs (NSAIDs) including ibuprofen and naprosyn. While banned in many European countries due to concerns about surgical bleeding and stomach ulcers after surgery, ketorolac is the only injectable NSAID in the United States.
In 2005, the U.S. Food and Drug Administration (FDA) issued a letter of non-approval for parecoxib in the United States. No reasons were ever documented publicly for the non-approval, although one study noted increased occurrences of heart attacks following cardiac bypass surgery compared to placebo when high doses of parecoxib were used to control pain after surgery. It is also important to remember that rare but severe allergic reactions (Stevens-Johnson Syndrome, Lyell Syndrome) have been described with valdecoxib, the molecule to which parecoxib is converted. The drug is not approved for use after cardiac surgery in Europe. Ketorolac, still banned in much of Europe, and IV Ibuprofen are therefore the only options for IV NSAIDs in the United States, and it is not clear whether parecoxib will be resubmitted to the FDA in the future.
...
Wikipedia
