OxyContin
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | many |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682132 |
| Pregnancy category |
|
| Dependence liability |
High |
| Routes of administration |
By mouth, sublingual, intramuscular, intravenous, intranasal, subcutaneous, transdermal, rectal, epidural |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 60–87% |
| Protein binding | 45% |
| Metabolism | Hepatic: mainly CYP3A, and, to a much lesser extent, CYP2D6 to oxymorphone (~5%); 95% metabolized (i.e., 5% excreted unchanged) |
| Onset of action | 10–30 minutes (IR) 1 hour (CR) |
| Biological half-life | 2–3 hours (IR) (variable) (same t1/2 for all ROAs) 4.5 hours (CR) |
| Duration of action | 3–6 hours (IR) (variable) 12 hours (CR) |
| Excretion | Urine (83%) |
| Identifiers | |
|
|
| Synonyms | Eukodal, eucodal; dihydrohydroxycodeinone, 7,8-dihydro-14-hydroxycodeinone, 6-deoxy-7,8-dihydro-14-hydroxy-3-O-methyl-6-oxomorphine |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.000.874 |
| Chemical and physical data | |
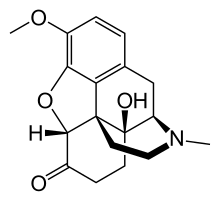
| Formula | C18H21NO4 |
| Molar mass | 315.364 g/mol |
| 3D model (Jmol) | |
| Solubility in water | HCl: 166 mg/mL (20 °C) |
|
|
|
|
Oxycodone is a semisynthetic opioid synthesized from thebaine, an opioid alkaloid found in the Persian poppy, and one of the many alkaloids found in the opium poppy. It is a moderately potent opioid analgesic, generally indicated for relief of moderate to severe pain. Oxycodone was developed in 1917 in Germany as one of several semi-synthetic opioids in an attempt to improve on the existing opioids.
Oxycodone is available as single-ingredient medication in immediate release and controlled release. Parenteral formulations of 10 mg/mL and 50 mg/mL are available in the UK for IV/IM administration. Combination products are also available as immediate-release formulations, with non-narcotic analgesic ingredients such as paracetamol (acetaminophen) and nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin and ibuprofen. An abuse-deterrent combination with naloxone is available in managed-release tablets. If injected, the naloxone precipitates opioid withdrawal symptoms and blocks the effect of the medication.
Oxycodone has been in clinical use since 1916, and it is used for managing moderate to moderately severe acute or chronic pain. It has been found to improve quality of life for those with many types of pain. Experts are divided regarding use for non-cancer-related chronic pain, as most opioids have great potential for dependence and may also create paradoxical pain sensitivity.
Oxycodone is available as controlled-release tablet, intended to be taken every 12 hours. A 2006 review found that controlled-release oxycodone is comparable to instant-release oxycodone, morphine, and hydromorphone in management of moderate to severe cancer pain, with fewer side effects than morphine. The author concluded that the controlled release form is a valid alternative to morphine and a first-line treatment for cancer pain. In 2014, the European Association for Palliative Care recommended oral oxycodone as a second-line alternative to oral morphine for cancer pain.
...
Wikipedia
