Osimertinib
 |
|
| Clinical data | |
|---|---|
| Trade names | Tagrisso, Tagrix |
| AHFS/Drugs.com | tagrisso |
| Routes of administration |
Oral tablets |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | Probably high |
| Metabolism | Oxidation (CYP3A) |
| Biological half-life | 48 hours |
| Excretion | Feces (68%), urine (14%) |
| Identifiers | |
|
|
| Synonyms | AZD9291 |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
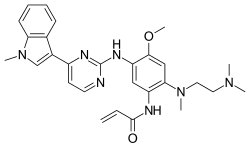
| Formula | C28H33N7O2 |
| Molar mass | 499.62 g·mol−1 |
| 3D model (Jmol) | |
|
|
|
|
Osimertinib (previously known as mereletinib or AZD9291; trade name Tagrisso) is a third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) drug developed by AstraZeneca Pharmaceuticals – for mutated EGFR cancers.
Osimertinib is used to treat locally advanced or metastatic non-small-cell lung cancer (NSCLC), when the cancer has the specific T790M mutation in the gene coding for epidermal growth factor receptor. In the US the mutation must be detected with an FDA-approved companion diagnostic test, and the person must have been stopped responding to the other EGFR-inhibitors.
The NSCLC in people receiving the drug has developed resistance mutations, namely C797S occurs in EGFR exon 20 or amplifications of the EGFR gene, even as T790M clones have been eliminated.
It causes fetal harm, so should not be used in women who are pregnant, and women who take it should avoid becoming pregnant.
Caution should be taken in people with a history of interstitial lung disease (ILD) were excluded from clinical trials, as the drug can cause severe ILD or pneumonia. Caution should also be taken in people with a predisposition to long QT syndrome as the drug can provoke this.
Very common (greater than 10% of clinical trial subjects) adverse effects include diarrhea, stomatitis, rashes, dry or itchy skin, infections where finger or toenails abut skin, low platelet counts, low leukocyte counts, and low neutrophil counts.
Common (between 1% and 10% of clinical trial subjects) adverse effects include interstitial lung disease.
Osimertinib is metabolized by CYP3A4 and CYP3A5, so substances that strongly inhibit either enyzme, like macrolide antibiotics, antifungals, and antivirals may increase exposure to osimertinib, and substances like rifampicin that activate either enzyme will decrease the effectiveness of osimertinib.
...
Wikipedia
