Nefazodone
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Dutonin, Nefadar, Rulivan, Serzone |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a695005 |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 20% (variable) |
| Protein binding | >99% |
| Metabolism | Hepatic (active metabolites, including mCPP) |
| Biological half-life | 2–4 hours |
| Excretion | Urine (55%), Feces (20–30%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
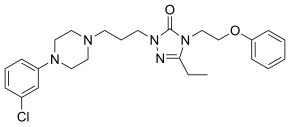
| Formula | C25H32ClN5O2 |
| Molar mass | 470.01 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Nefazodone (Dutonin, Nefadar, Serzone) is an antidepressant marketed by Bristol-Myers Squibb. Its sale was discontinued in 2003 in some countries due to the rare incidence of hepatotoxicity (liver damage), which could lead to the need for a liver transplant, or even death. The incidence of severe liver damage is approximately 1 in every 250,000 to 300,000 patient-years. On June 14, 2004, Bristol-Myers Squibb discontinued the sale of Serzone in the United States and Canada. Several generic formulations of nefazodone are still available.
Nefazodone acts primarily as a potent antagonist at the 5-HT2A receptors (Kd 26 nM). It also has moderate affinity for the α1-adrenergic receptor (Kd 48 nM) and 5-HT1A receptor (Kd 80 nM), and very low affinity for the α2-adrenergic receptor (Kd 640 nM) and D2 receptor (Kd 910 nM). Nefazodone has low affinity for the serotonin (200 nM), norepinephrine (360 nM), and dopamine (360 nM) transporters as well, and therefore acts as a weak serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI). It has negligible affinity for the H1 receptor (24,000 nM) or muscarinic acetylcholine receptors (11,000 nM), and accordingly lacks any antihistamine or anticholinergic side effects.
...
Wikipedia
