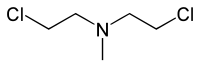
Mechlorethamine
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
2-Chloro-N-(2-chloroethyl)-N-methylethan-1-amine
|
|
| Other names
Bis(2-chloroethyl)(methyl)amine
|
|
| Identifiers | |
|
51-75-2 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:28925 |
| ChEMBL |
ChEMBL427 |
| ChemSpider |
3893 |
| DrugBank |
DB00888 |
| ECHA InfoCard | 100.000.110 |
| EC Number | 200-120-5 |
| KEGG |
D0767 |
| MeSH | Mechlorethamine |
| PubChem | 4033 |
| RTECS number | IA1750000 |
| UNII |
50D9XSG0VR |
| UN number | 2810 |
|
|
|
|
| Properties | |
| C5H11Cl2N | |
| Molar mass | 156.05 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Fishy, ammoniacal |
| log P | 0.91 |
| Pharmacology | |
| D08AX04 (WHO) L01AA05 (WHO) | |
|
|
|
|
| Pharmacokinetics: | |
| <1 minute | |
| 50% (renal) | |
| Legal status |
|
| Related compounds | |
|
Related amines
|
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Chlormethine (INN, BAN), mechlorethamine (widely used in the US, not the USAN, however) also known as mustine and HN2 and in former USSR known as Embichin is a nitrogen mustard sold under the brand name Mustargen. It is the prototype of alkylating agents, a group of anticancer chemotherapeutic drugs. It works by binding to DNA, crosslinking two strands and preventing cell duplication. It binds to the N7 nitrogen on the DNA base guanine. As the chemical is a blister agent, its use is strongly restricted within the Chemical Weapons Convention where it is classified as a Schedule 1 substance.
Mechlorethamine belongs to the group of nitrogen mustard alkylating agents.
It has been derivatized into the estrogen analogue estramustine phosphate, used to treat prostate cancer. It can also be used in chemical warfare where it has the code-name HN2. This chemical is a form of nitrogen mustard gas and a powerful vesicant. Historically, some uses of mechlorethamine have included lymphoid malignancies such as Hodgkin’s disease, lymphosarcoma, chronic myelocytic leukemia, polycythemia vera, and bronchogenic carcinoma Mechlorethamine is often administered intravenously, but when compounded into a topical formulation it can also be used to treat skin diseases. There have been studies demonstrating that topical administration of mechlorethamine has efficacy in mycosis fungoides-type cutaneous T cell lymphoma.
...
Wikipedia
