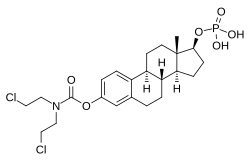
Estramustine phosphate
 |
|
| Clinical data | |
|---|---|
| Trade names | Emcyt, Estracyt |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608046 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 75% |
| Metabolism | Hepatic |
| Metabolites | Estramustine, estromustine, normustine, estradiol, estrone, phosphoric acid |
| Biological half-life | 15–24 hours |
| Excretion | Feces (2.9–4.8%) |
| Identifiers | |
|
|
| Synonyms | Leo 299; NSC-89199; Ro 21-8837/001; Estradiol 3-[bis(2-chloroethyl)carbamate] dihydrogen phosphate |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.019.161 |
| Chemical and physical data | |
| Formula | C23H32Cl2NO6P |
| Molar mass | 520.384 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Estramustine phosphate, sold under the brand names Emcyt and Estracyt and provided as the sodium or meglumine salt, is a dual alkylating antineoplastic agent (i.e., a chemotherapy drug) of the nitrogen mustard type and hormonal antineoplastic agent of the estrogen type that is used in the treatment of prostate cancer.
Estramustine phosphate is indicated, in the United States, for the palliative treatment of metastatic and/or progressive prostate cancer, whereas in the United Kingdom it is indicated for the treatment of unresponsive or relapsing prostate cancer.
Estramustine phosphate is contraindicated when used in children, patients hypersensitive to estrogen or nitrogen mustards, those with peptic ulcer (an ulcer in the digestive tract), those with severely compromised liver function, those with weak heart muscle (also known as myocardial insufficiency) and those with thromboembolic disorders or complications related to fluid retention.
...
Wikipedia
