Lead(II) oxide
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Lead(II) oxide
|
|
| Other names | |
| Identifiers | |
| ECHA InfoCard | 100.013.880 |
|
PubChem CID
|
|
| RTECS number | OG1750000 |
| UN number | 3288 |
| Properties | |
| PbO | |
| Molar mass | 223.20 g/mol |
| Appearance | red or yellow powder |
| Density | 9.53 g/cm3 |
| Melting point | 888 °C (1,630 °F; 1,161 K) |
| Boiling point | 1,477 °C (2,691 °F; 1,750 K) |
| 0.017 g/L | |
| Solubility | insoluble in dilute alkalis, alcohol soluble in concentrated alkalis soluble in HCl, ammonium chloride |
| −42.0·10−6 cm3/mol | |
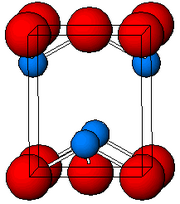
| Structure | |
| tetragonal, tP4 | |
| P4/nmm, No. 129 | |
| Hazards | |
| Safety data sheet | ICSC 0288 |
|
EU classification (DSD)
|
Repr. Cat. 1/3 Harmful (Xn) Dangerous for the environment (N) |
| R-phrases | R61, R20/22, R33, R62, R50/53 |
| S-phrases | S53, S45, S60, S61 |
| NFPA 704 | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
|
LDLo (lowest published)
|
1400 mg/kg (dog, oral) |
| Related compounds | |
|
Other anions
|
Lead sulfide Lead selenide Lead telluride |
|
Other cations
|
Carbon monoxide Silicon monoxide Tin(II) oxide |
|
Lead(II,II,IV) oxide Lead dioxide |
|
|
Related compounds
|
Thallium(III) oxide Bismuth(III) oxide |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Lead(II) oxide, also called lead monoxide, is the inorganic compound with the molecular formula PbO. PbO occurs in two polymorphs, one litharge having a tetragonal crystal structure and the other massicot having an orthorhombic crystal structure. Modern applications for PbO are mostly in lead-based industrial glass and industrial ceramics, including computer components.It is an amphoteric oxide.
PbO may be prepared by heating lead metal in air at approx. 600 °C. At this temperature it is also the end product of oxidation of other in air:
Thermal decomposition of lead(II) nitrate or lead carbonate also results in the PbO formation:
PbO is produced on a large scale as an intermediate product in refining raw lead ores into metallic lead. The usual lead ore is galena (lead(II) sulfide). At high temperature (1000 °C) the sulfide is converted to the oxide:
Metallic lead is obtained by reducing the PbO with carbon monoxide at around 1200 °C:.
As determined by X-ray crystallography, both polymorphs, tetragonal and orthorhombic feature a pyramidal four-coordinate Pb center. In the tetragonal form the four Pb-O bonds have the same length, but in the orthorhombic two are shorter and two longer. The pyramidal nature indicates the presence of a stereo-chemically active lone pair of electrons. When PbO occurs in tetragonal lattice structure it is called litharge; and when the PbO has orthorhombic lattice structure it is called massicot. The PbO can be changed from massicot to litharge or vice versa by controlled heating and cooling. The tetragonal form is usually red or orange color, while the orthorhombic is usually yellow or orange, but the color is not a very reliable indicator of the structure. The tetragonal and orthorhombic forms of PbO occur naturally as rare minerals.
...
Wikipedia

