Lead(II) sulfide
 |
|
 |
|
| Names | |
|---|---|
| Other names
Plumbous sulfide
Galena, Sulphuret of lead |
|
| Identifiers | |
|
1314-87-0 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
14135 |
| ECHA InfoCard | 100.013.861 |
|
|
|
|
| Properties | |
| PbS | |
| Molar mass | 239.30 g/mol |
| Density | 7.60 g/cm3 |
| Melting point | 1,118 °C (2,044 °F; 1,391 K) |
| Boiling point | 1,281 °C (2,338 °F; 1,554 K) |
| 2.6×10−11 kg/kg (calculated, at pH=7) 8.6×10−7 kg/kg | |
|
Solubility product (Ksp)
|
9.04×10−29 |
| −84.0·10−6 cm3/mol | |
|
Refractive index (nD)
|
3.91 |
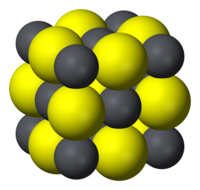
| Structure | |
| Halite (cubic), cF8 | |
| Fm3m, No. 225 | |
|
a = 5.936 Angstroms
|
|
| Octahedral (Pb2+) Octahedral (S2−) |
|
| Thermochemistry | |
| 46.02 J/degree mol | |
|
Std molar
entropy (S |
91.3 J/mol |
|
Std enthalpy of
formation (ΔfH |
–98.7 kJ/mol |
| Hazards | |
| Safety data sheet | External MSDS |
|
EU classification (DSD)
|
Repr. Cat. 1/3 Harmful (Xn) Dangerous for the environment (N) |
| R-phrases | R61, R20/22, R33, R62, R50/53 |
| S-phrases | S53, S45, S60, S61 |
| NFPA 704 | |
| Flash point | Non-flammable |
| Related compounds | |
|
Other anions
|
Lead(II) oxide Lead selenide Lead telluride |
|
Other cations
|
Carbon monosulfide Silicon monosulfide Germanium(II) sulfide Tin(II) sulfide |
|
Related compounds
|
Thallium sulfide Lead(IV) sulfide Bismuth sulfide |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Lead(II) sulfide (also spelled sulphide) is an inorganic compound with the formula PbS. PbS, also known as galena, is the principal ore, and most important compound of lead. It is a semiconducting material with niche uses.
Addition of hydrogen sulfide or sulfide salts to a solution of lead ions gives PbS as an insoluble black precipitate.
The equilibrium constant for this reaction is 3×106 M. This reaction, which entails a dramatic color change from colourless or white to black, was once used in qualitative inorganic analysis. The presence of hydrogen sulfide or sulfide ions is still routinely tested using "lead acetate paper."
Like the related materials PbSe and PbTe, PbS is a semiconductor. In fact, lead sulfide was one of the earliest materials to be used as a semiconductor. Lead sulfide crystallizes in the sodium chloride motif, unlike many other IV-VI semiconductors.
Since PbS is the main ore of lead, much effort has focused on its conversion. A major process involves smelting of PbS followed by reduction of the resulting oxide. Idealized equations for these two steps are:
The sulfur dioxide is converted to sulfuric acid.
Lead sulfide-containing nanoparticle and quantum dots have been well studied. Traditionally, such materials are produced by combining lead salts with a variety of sulfide sources. PbS nanoparticles have been recent examined for use in solar cells.
...
Wikipedia

