Lead(II) chloride
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC names
Lead(II) chloride
Lead dichloride |
|
| Other names
Plumbous chloride
Cotunnite |
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.028.950 |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| PbCl2 | |
| Molar mass | 278.10 g/mol |
| Appearance | white odorless solid |
| Density | 5.85 g/cm3 |
| Melting point | 501 °C (934 °F; 774 K) |
| Boiling point | 950 °C (1,740 °F; 1,220 K) |
| 10.8 g/L (20 °C) | |
|
Solubility product (Ksp)
|
5.89×10−5 (20 °C) |
| Solubility | slightly soluble in dilute HCl, ammonia; insoluble in alcohol |
| −73.8·10−6 cm3/mol | |
|
Refractive index (nD)
|
2.199 |
| Structure | |
| Orthorhombic, oP12 | |
| Pnma, No. 62 | |
| Thermochemistry | |
|
Std molar
entropy (S |
135.98 J K−1 mol−1 |
|
Std enthalpy of
formation (ΔfH |
-359.41 kJ/mol |
| Hazards | |
| Safety data sheet | See: data page |
|
EU classification (DSD)
|
|
| R-phrases | R61, R20/22, R33, R62, R50/53 |
| S-phrases | S53, S45, S60, S61 |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
|
LDLo (lowest published)
|
1500 mg/kg (guinea pig, oral) |
| Related compounds | |
|
Other anions
|
Lead(II) fluoride Lead(II) bromide Lead(II) iodide |
|
Other cations
|
Lead(IV) chloride Tin(II) chloride Germanium(II) chloride |
|
Related compounds
|
Thallium(I) chloride Bismuth chloride |
| Supplementary data page | |
|
Refractive index (n), Dielectric constant (εr), etc. |
|
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Lead(II) chloride (PbCl2) is an inorganic compound which is a white solid under ambient conditions. It is poorly soluble in water. Lead(II) chloride is one of the most important lead-based reagents. It also occurs naturally in the form of the mineral cotunnite.
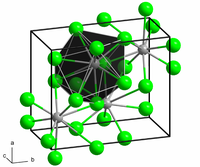
In solid PbCl2, each lead ion is coordinated by 9 chloride ions — 6 lie at the apices of a trigonal prism and 3 lie beyond the centers of each prism face. The 9 chloride ions are not equidistant from the central lead atom, 7 lie at 280–309 pm and 2 at 370 pm. PbCl2 forms white orthorhombic needles. While Lead(II) chloride is abundant in many natural water reserves, it is unsafe for human consumption and must be filtered out.
Vaporized PbCl2 molecules have a bent structure with the Cl-Pb-Cl angle being 98° and each Pb-Cl bond distance being 2.44 Å. Such PbCl2 is emitted from internal combustion engines that use ethylene chloride-tetraethyllead additives for antiknock purposes.
The solubility of PbCl2 in water is low (10.8 g/L at 20 °C) and for practical purposes it is considered insoluble. Its solubility product constant (Ksp) is 5.89×10−5. It is one of only four commonly insoluble chlorides, the other three being silver chloride (AgCl) with Ksp = 1.8×10−10, copper(I) chloride (CuCl) with Ksp = 1.72×10−7 and mercury(I) chloride (Hg2Cl2) with Ksp = 1.3×10−18.
PbCl2 occurs naturally in the form of the mineral cotunnite. It is colorless, white, yellow, or green with a density of 5.3–5.8 g/cm3. The hardness on the Mohs scale is 1.5–2. The crystal structure is orthorhombic dipyramidal and the point group is 2/m 2/m 2/m. Each Pb has a coordination number of 9. Cotunnite occurs near volcanoes: Vesuvius, Italy; Tarapacá, Chile; and Tolbachik, Russia.
...
Wikipedia

