Copper(I) chloride
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Copper(I) chloride
|
|
| Other names
Cuprous chloride
|
|
| Identifiers | |
|
7758-89-6 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:53472 |
| ChemSpider |
56403 |
| ECHA InfoCard | 100.028.948 |
| EC Number | 231-842-9 |
| PubChem | 62652 |
| RTECS number | GL6990000 |
|
|
|
|
| Properties | |
| CuCl | |
| Molar mass | 98.999 g/mol |
| Appearance | white powder, slightly green from oxidized impurities |
| Density | 4.145 g/cm3 |
| Melting point | 426 °C (799 °F; 699 K) |
| Boiling point | 1,490 °C (2,710 °F; 1,760 K) (decomposes) |
| 0.0062 g/100 mL (20 °C) | |
|
Solubility product (Ksp)
|
1.72 x 10−7 |
| Solubility | insoluble in ethanol acetone; soluble in concentrated HCl, NH4OH |
| -40.0·10−6 cm3/mol | |
|
Refractive index (nD)
|
1.930 |
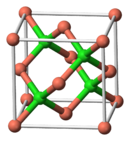
| Structure | |
| Zinc blende structure | |
| Hazards | |
| Safety data sheet | JT Baker |
|
EU classification (DSD)
|
Harmful (Xn) Dangerous for the environment (N) |
| R-phrases | R22, R50/53 |
| S-phrases | (S2), S22, S60, S61 |
| NFPA 704 | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
140 mg/kg |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
TWA 1 mg/m3 (as Cu) |
|
REL (Recommended)
|
TWA 1 mg/m3 (as Cu) |
|
IDLH (Immediate danger)
|
TWA 100 mg/m3 (as Cu) |
| Related compounds | |
|
Other anions
|
Copper(I) bromide Copper(I) iodide |
|
Other cations
|
Copper(II) chloride Silver(I) chloride |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear green due to the presence of copper(II) chloride.
Copper(I) chloride was first prepared by Robert Boyle in the mid-seventeenth century from mercury(II) chloride ("Venetian sublimate") and copper metal:
In 1799, J.L. Proust characterized the two different chlorides of copper. He prepared CuCl by heating CuCl2 at red heat in the absence of air, causing it to lose half of its combined chlorine followed by removing residual CuCl2 by washing with water.
An acidic solution of CuCl was formerly used for analysis of carbon monoxide content in gases, for example in Hempel's gas apparatus. This application was significant during the time that coal gas was widely used for heating and lighting, during the nineteenth and early twentieth centuries.
Copper(I) chloride is produced industrially by the direct combination of copper metal and chlorine at 450–900 °C:
Cu + 0.5 Cl2 → CuCl
Copper(I) chloride can also be prepared by reducing copper(II) chloride, e.g. with sulfur dioxide:
2 CuCl2 + SO2 + 2 H2O → 2 CuCl + H2SO4 + 2 HCl
Many other reducing agents can be used.
Copper(I) chloride is a Lewis acid, which is classified as soft according to the Hard-Soft Acid-Base concept. Thus, it tends to form stable complexes with soft Lewis bases such as triphenylphosphine:
Although CuCl is insoluble in water, it dissolves in aqueous solutions containing suitable donor molecules. It forms complexes with halide ions, for example forming H3O+ CuCl2− with concentrated hydrochloric acid. It is attacked by CN−, S2O32−, and NH3 to give the corresponding complexes.
...
Wikipedia

