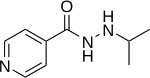
Iproniazid
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration |
? |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Biological half-life | ? |
| Excretion | ? |
| Legal status | |
| Legal status |
|
| ECHA InfoCard | 100.000.199 |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C9H13N3O |
| Molar mass | 179.219 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Iproniazid (Marsilid, Rivivol, Euphozid, Iprazid, Ipronid, Ipronin) is a non-selective, irreversible monoamine oxidase inhibitor (MAOI) of the hydrazine class. It was discontinued in most of the world in the 1960s, but remained in use in France until fairly recently.
Iproniazid was originally developed for the treatment of tuberculosis, but in 1952, its antidepressant properties were discovered when researchers noted that patients given isoniazid became inappropriately happy. Subsequently N-isopropyl addition led to development as an antidepressant and was approved for use in 1958. It was withdrawn a few years later in 1961 due to a high incidence of hepatitis, and was replaced by less hepatotoxic drugs such as phenelzine and isocarboxazid.
Although iproniazid was one of the first antidepressants ever marketed, amphetamine (marketed as Benzedrine from 1935, for "mild depression", amid other indications) predates it; and frankincense has been marketed traditionally for millennia for, among other things, altering mood, although it was not until 2012 that one of the components of its smoke was found to have antidepressant effects in mice.
...
Wikipedia
