Benzedrine
 |
|
 |
|
| Clinical data | |
|---|---|
| Pronunciation | |
| Trade names | Adderall, Dyanavel XR, Evekeo, others |
| AHFS/Drugs.com | amphetamine |
| License data |
|
| Pregnancy category |
|
| Dependence liability |
Physical: none Psychological: moderate |
| Addiction liability |
Moderate |
| Routes of administration |
Medical: oral, intravenous Recreational: oral, insufflation, rectal, intravenous, intramuscular |
| Drug class | CNS stimulant |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral 75–100% |
| Protein binding | 15–40% |
| Metabolism | CYP2D6,DBH,FMO3 |
| Metabolites | 4-hydroxyamphetamine, 4-hydroxynorephedrine, 4-hydroxyphenylacetone, benzoic acid, hippuric acid, norephedrine, phenylacetone |
| Onset of action |
IR dosing: 30–60 minutes XR dosing: 1.5–2 hours |
| Biological half-life |
D-amph: 9–11 hours L-amph: 11–14 hours pH-dependent: 8–31 hours |
| Duration of action |
IR dosing: 3–7 hours XR dosing: 12 hours |
| Excretion | Primarily renal; pH-dependent range: 1–75% |
| Identifiers | |
|
|
| Synonyms | α-methylphenethylamine |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| ECHA InfoCard | 100.005.543 |
| Chemical and physical data | |
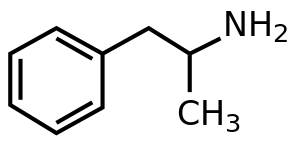
| Formula | C9H13N |
| Molar mass | 135.20622 g/mol |
| 3D model (Jmol) | |
| Chirality | Racemic mixture |
| Density | .913 g/cm3 at 25 °C |
| Melting point | 11.3 °C (52.3 °F) (predicted) |
| Boiling point | 203 °C (397 °F) at 760 mmHg |
|
|
|
|
Amphetamine (contracted from alpha‑methylphenethylamine) is a potent central nervous system (CNS) stimulant that is used in the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy, and obesity. Amphetamine was discovered in 1887 and exists as two enantiomers:levoamphetamine and dextroamphetamine. Amphetamine properly refers to a specific chemical, the racemic free base, which is equal parts of the two enantiomers, levoamphetamine and dextroamphetamine, in their pure amine forms. The term is frequently used informally to refer to any combination of the enantiomers, or to either of them alone. Historically, it has been used to treat nasal congestion and depression. Amphetamine is also used as an athletic performance enhancer and cognitive enhancer, and recreationally as an aphrodisiac and euphoriant. It is a prescription drug in many countries, and unauthorized possession and distribution of amphetamine are often tightly controlled due to the significant health risks associated with recreational use.
The first pharmaceutical amphetamine was Benzedrine, a brand which was used to treat a variety of conditions. Currently, pharmaceutical amphetamine is prescribed as racemic amphetamine, Adderall,dextroamphetamine, or the inactive prodrug lisdexamfetamine. Amphetamine, through activation of a trace amine receptor, increases monoamine and excitatory neurotransmitter activity in the brain, with its most pronounced effects targeting the catecholamine neurotransmitters norepinephrine and dopamine.
...
Wikipedia
