Iclusig
 |
|
 |
|
| Clinical data | |
|---|---|
| Pronunciation | /poʊˈnætɪnɪb/ poh-NAT-i-nib |
| Trade names | Iclusig |
| License data | |
| Pregnancy category |
|
| Routes of administration |
By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Unknown |
| Protein binding | >99% (in vitro) |
| Metabolism | Liver (CYP3A4, 2C8, 2D6, 3A5) |
| Biological half-life | 12–66 hours |
| Excretion | Feces (87%), urine (5%) |
| Identifiers | |
|
|
| Synonyms | AP24534 |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
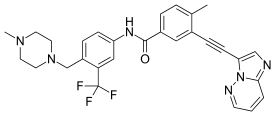
| Formula | C29H27F3N6O |
| Molar mass | 532.56 g/mol |
| 3D model (JSmol) | |
|
|
|
|
Ponatinib (trade name Iclusig /aɪˈkluːsɪɡ/ eye-KLOO-sig, previously AP24534) is an oral drug developed by ARIAD Pharmaceuticals for the treatment of chronic myeloid leukemia (CML) and Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL). It is a multi-targeted tyrosine-kinase inhibitor. Some forms of CML, those that have the T315I mutation, are resistant to current therapies such as imatinib. Ponatinib has been designed to be effective against these types of tumors.
The United States Food and Drug Administration approved the drug as a candidate in December 2012, but temporarily suspended sales on 31 October 2013 because of "the risk of life-threatening blood clots and severe narrowing of blood vessels". This suspension was partially lifted on Dec. 20, 2013 with ponatinib being issued revised prescribing information, a new "Black Box Warning" and a "Risk Evaluation and Mitigation Strategy" in place to better evaluate the risks and benefits of using the drug.
In the US it can cost $138,000 a year, which has been criticized.
Ponatinib was approved by the US FDA on December 14, 2012, for patients with resistant or intolerant CML and Ph+ ALL, based on results of the PACE phase II trial reported days earlier at the annual ASH meeting. Because the approval was under the FDA Accelerated Approval program the applicant was required to carry out additional studies. Based on these additional studies, the FDA granted in 2016 full approval and label update to ponatinib (Iclusig) for patients with chronic phase, accelerated phase, or blast phase chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia for whom no other tyrosine kinase inhibitor therapy is indicated. Approval was also granted for T315I-positive and T315I-positive Philadelphia chromosome positive acute lymphoblastic leukemia.
...
Wikipedia
