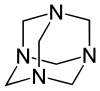
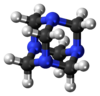
Hexamine
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
1,3,5,7-Tetraazatricyclo[3.3.1.13,7]decane
|
|||
| Other names
Hexamine; Methenamine;
Urotropine; 1,3,5,7- tetraazaadamantane, Formin, Aminoform |
|||
| Identifiers | |||
|
100-97-0 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChEBI |
CHEBI:6824 |
||
| ChEMBL |
ChEMBL1201270 |
||
| ChemSpider |
3959 |
||
| ECHA InfoCard | 100.002.642 | ||
| EC Number | 202-905-8 | ||
| E number | E239 (preservatives) | ||
| KEGG |
D00393 |
||
| MeSH | Methenamine | ||
| PubChem | 4101 | ||
| UNII |
J50OIX95QV |
||
|
|||
|
|||
| Properties | |||
| C6H12N4 | |||
| Molar mass | 140.186 g/mol | ||
| Appearance | White crystalline solid | ||
| Density | 1.33 g/cm3 (at 20 °C) | ||
| Melting point | 280 °C (536 °F; 553 K) (sublimes) | ||
| 85.3 g/100 mL | |||
| Acidity (pKa) | 4.89 | ||
| Pharmacology | |||
| J01XX05 (WHO) | |||
| Hazards | |||
| Main hazards | Highly flammable, harmful | ||
| Flash point | 250 °C (482 °F; 523 K) | ||
| 410 °C (770 °F; 683 K) | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Hexamethylenetetramine or methenamine is a heterocyclic organic compound with the formula (CH2)6N4. This white crystalline compound is highly soluble in water and polar organic solvents. It has a cage-like structure similar to adamantane. It is useful in the synthesis of other chemical compounds, e.g., plastics, pharmaceuticals, rubber additives. It sublimes in vacuum at 280 °C.
The compound was discovered by Aleksandr Butlerov in 1859.
Hexamethylenetetramine is prepared industrially by combining formaldehyde and ammonia. The reaction can be conducted in gas phase and in solution.
The molecule has a symmetric tetrahedral cage-like structure, similar to adamantane, whose four "corners" are nitrogen atoms and "edges" are methylene bridges. Although the molecular shape defines a cage, no void space is available at the interior for binding other atoms or molecules, unlike crown ethers or larger cryptand structures.
The molecule behaves like an amine base, undergoing protonation and N-alkylation.
The dominant use of hexamethylenetetramine is in the production of powdery or liquid preparations of phenolic resins and phenolic resin moulding compounds, where it is added as a hardening component. These products are used as binders, e.g. in brake and clutch linings, abrasive products, non-woven textiles, formed parts produced by moulding processes, and fireproof materials.
It has been proposed that hexamethylenetetramine could work as a molecular building block for self-assembled molecular crystals.
As the mandelic acid salt (generic methenamine mandelate, USP) it is used for the treatment of urinary tract infection. It decomposes at an acidic pH to form formaldehyde and ammonia, and the formaldehyde is bactericidal; the mandelic acid adds to this effect. Urinary acidity is typically ensured by co-administering vitamin C (ascorbic acid) or ammonium chloride. Its use had temporarily been reduced in the late 1990s, due to adverse effects, particularly chemically-induced hemorrhagic cystitis in overdose, but its use has now been re-approved because of the prevalence of antibiotic resistance to more commonly used drugs. This drug is particularly suitable for long-term prophylactic treatment of urinary tract infection, because bacteria do not develop resistance to formaldehyde. It should not be used in the presence of renal insufficiency. Methenamine in the form of cream and spray is successfully used for treatment of excessive sweating and concomitant odour.
...
Wikipedia