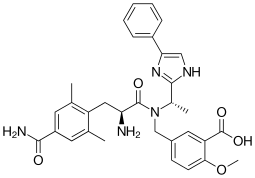
Eluxadoline
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Viberzi |
| Routes of administration |
Oral |
| ATC code | A07DA06 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 81% |
| Biological half-life | 3.7–6 hours |
| Excretion | 82.2% (feces), <1% (urine) |
| Identifiers | |
|
|
| CAS Number | 864821-90-9 |
| PubChem (CID) | 11250029 |
| IUPHAR/BPS | 7691 |
| ChemSpider | 9425062 |
| UNII | 45TPJ4MBQ1 |
| KEGG | D10403 |
| Chemical and physical data | |
| Formula | C32H35N5O5 |
| Molar mass | 569.6508 g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
Eluxadoline (INN, USAN) (brand name Viberzi vye-BER-zee; former developmental code name JNJ-27018966) is an orally-active drug approved for the treatment of diarrhea and abdominal pain in individuals with diarrhea-predominant irritable bowel syndrome (IBS-D). It was approved for use by the United States Food and Drug Administration on May 27, 2015. The drug originated from Janssen Pharmaceutica and was developed by Actavis.
This drug is contraindicated in case of having:
Common adverse effects in the two phase III clinical trials were constipation and nausea but rates of discontinuation due to constipation were low for both eluxadoline and placebo. Rare adverse effects: fatigue, bronchitis, viral gastroenteritis. Rare serious adverse effect in a clinical trial was pancreatitis with a general incidence of 0.3% - higher incidence with 100 mg dose (0.3%) than with 75 mg dose (0.2%).
Elevated concentrations of eluxadoline were observed with coadministraion of inhibitors of the transporter protein OATP1B1, such as:
Also, concurrent use of other drugs that cause constipation is not preferred, such as:
Eluxadoline increases the concentrations of drugs which are OATP1B1 and BCRP substrates. Also, coadministration of eluxadoline with rosuvastatin may increase the risk of rhabdomyolysis.
Eluxadoline is a μ- and κ-opioid receptor agonist and δ-opioid receptor antagonist that acts locally in the enteric nervous system, possibly decreasing adverse effects on the central nervous system.
...
Wikipedia
