Dipipanone
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Diconal |
| Routes of administration |
Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver-mediated |
| Biological half-life | 3.5 hours |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.006.728 |
| Chemical and physical data | |
| Formula | C24H31NO |
| Molar mass | 349.52 |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Dipipanone (Pipadone) is a strong opioid analgesic drug, used for very severe pain in cases where other analgesics are unsuitable, for instance where morphine is indicated but cannot be used due to the patient being allergic to morphine.
The main preparation of the drug commercially available is mixed with cyclizine (Diconal, Wellconal) which has the advantage of reducing nausea, vomiting and histamine release associated with strong opioid therapy.
Dipipanone was also available as an oral mixture 10 mg/5ml without the cyclizine during the 1970s–1980s in the United Kingdom. This form was rare and used normally only in drug trials and in specialist diconal addiction clinics.
With the exception of oxycodone, dipipanone is now the only alternative opioid left to use in the UK that is of equal strength to morphine that can be prescribed instead. All the other opioids are either weaker like codeine, dihydrocodeine and pethidine or stronger like hydromorphone, methadone, and fentanyl.
One of the biggest disadvantages of using dipipanone is that it is only produced in one dosage form that is mixed with the anti-emetic cyclizine at a ratio of 25% dipipanone to 75% cyclizine which limits the dose of dipipanone to an absolute maximum of 3 tablets per dose up to 4-6 times a day.
As of November 2011 Amdipharm stopped making the Diconal brand tablets for the UK due to undisclosed commercial reasons. However the product is listed as available on the manufacturer's website as of July 1, 2014. General practitioners are now advised to prescribe it as generic dipipanone/cyclizine tablets.
Dipipanone is now unavailable in most countries of the world either by laws prohibiting its medicinal use as in the United States or by falling out of production as more modern analgesics took its market share. Great Britain, Northern Ireland and South Africa are known to continue to use the substance but it is infrequently prescribed.
Dipipanone is a Schedule I controlled substance in the United States; it has been assigned the ACSCN of 9622 and in 2013 had an annual manufacturing quota of 5 g.
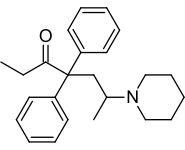
Chemically, dipipanone belongs to the class of opioids called the 4,4-diphenylheptane-3-ones. It closely resembles methadone, the only structural difference being the N,N-dimethyl moiety of methadone being replaced with a piperidine ring. Other related compounds with equivalent activity where the piperidine ring has been replaced by other groups, include the morpholine derivative phenadoxone, as well as the corresponding pyrrolidine derivative.
...
Wikipedia
