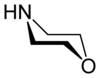
Morpholine
|
|
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Morpholine
|
|||
| Other names
Diethylenimide oxide
1,4-Oxazinane Tetrahydro-1,4-oxazine Diethylene imidoxide Diethylene oximide Tetrahydro-p-oxazine |
|||
| Identifiers | |||
|
110-91-8 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChEBI |
CHEBI:34856 |
||
| ChEMBL |
ChEMBL276518 |
||
| ChemSpider |
13837537 |
||
| ECHA InfoCard | 100.003.469 | ||
| KEGG |
C14452 |
||
| RTECS number | QD6475000 | ||
| UNII |
8B2ZCK305O |
||
|
|||
|
|||
| Properties | |||
| C4H9NO | |||
| Molar mass | 87.12 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Weak ammonia-like or fish-like | ||
| Density | 1.007 g/cm3 | ||
| Melting point | −5 °C (23 °F; 268 K) | ||
| Boiling point | 129 °C (264 °F; 402 K) | ||
| miscible | |||
| Vapor pressure | 6 mmHg (20 °C) | ||
| Acidity (pKa) | 8.36 (of conjugate acid) | ||
| -55.0·10−6 cm3/mol | |||
| Hazards | |||
| Main hazards | Flammable, Corrosive | ||
| Safety data sheet | hazard.com | ||
| R-phrases | R10 R20/21/22 R34 | ||
| S-phrases | (S1/2) S23 S36 S45 | ||
| NFPA 704 | |||
| Flash point | 31 °C (88 °F; 304 K) | ||
| 275 °C (527 °F; 548 K) | |||
| Explosive limits | 1.4%–11.2% | ||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
1220 mg/kg (mammal, oral) 525 mg/kg (mouse, oral) 1050 mg/kg (rat, oral) |
||
|
LC50 (median concentration)
|
365 ppm (mouse, 2 hr) | ||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
TWA 20 ppm (70 mg/m3) [skin] | ||
|
REL (Recommended)
|
TWA 20 ppm (70 mg/m3) ST 30 ppm (105 mg/m3) [skin] | ||
|
IDLH (Immediate danger)
|
1400 ppm | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Morpholine is an organic chemical compound having the chemical formula O(CH2CH2)2NH. This heterocycle features both amine and ether functional groups. Because of the amine, morpholine is a base; its conjugate acid is called morpholinium. For example, treating morpholine with hydrochloric acid makes the salt morpholinium chloride.
Morpholine may be produced by the dehydration of diethanolamine with sulfuric acid:
Morpholine is a common additive, in parts per million concentrations, for pH adjustment in both fossil fuel and nuclear power plant steam systems. Morpholine is used because its volatility is about the same as water, so once it is added to the water, its concentration becomes distributed rather evenly in both the water and steam phases. Its pH-adjusting qualities then become distributed throughout the steam plant to provide corrosion protection. Morpholine is often used in conjunction with low concentrations of hydrazine or ammonia to provide a comprehensive all-volatile treatment chemistry for corrosion protection for the steam systems of such plants. Morpholine decomposes reasonably slowly in the absence of oxygen at the high temperatures and pressures in these steam systems.
...
Wikipedia