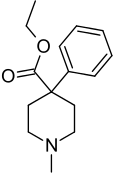
Pethidine
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Demerol |
| Pregnancy category |
|
| Dependence liability |
High |
| Routes of administration |
oral, intravenous, intramuscular, subcutaneous |
| ATC code | N02AB02 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 50–60% (Oral), 80-90% (Oral, in cases of hepatic impairment) |
| Protein binding | 65-75% |
| Metabolism | Liver |
| Biological half-life | 2.5-4 hours, 7-11 hours (liver disease) |
| Excretion | Renal |
| Identifiers | |
|
|
| Synonyms | meperidine |
| CAS Number |
57-42-1 |
| PubChem (CID) | 4058 |
| IUPHAR/BPS | 7221 |
| DrugBank |
DB00454 |
| ChemSpider |
3918 |
| UNII |
9E338QE28F |
| KEGG |
D08343 |
| ChEMBL |
CHEMBL607 |
| ECHA InfoCard | 100.000.299 |
| Chemical and physical data | |
| Formula | C15H21NO2 |
| Molar mass | 247.33g/mol |
| 3D model (Jmol) | Interactive image |
|
|
|
|
Pethidine, also known as meperidine and Demerol, is a synthetic opioid pain medication of the phenylpiperidine class. Synthesized in 1939 as a potential anticholinergic agent by the German chemist Otto Eisleb, its analgesic properties were first recognized by Otto Schaumann while working for IG Farben, Germany. Pethidine was the first wholly synthetic opioid developed and is the prototype of a large family of analgesics including the pethidine 4-phenylpiperidines (pethidine, piminodine, anileridine and others), the prodines (alphaprodine, MPPP, etc.), bemidones (ketobemidone, etc.) and others more distant, including diphenoxylate and analogues.
Pethidine is indicated for the treatment of moderate to severe pain, and is delivered as a hydrochloride salt in tablets, as a syrup, or by intramuscular, subcutaneous, or intravenous injection. For much of the 20th century, pethidine was the opioid of choice for many physicians; in 1975, 60% of doctors prescribed it for acute pain and 22% for chronic severe pain.
Compared with morphine, pethidine was thought to be safer, carry a lower risk of addiction, and to be superior in treating the pain associated with biliary spasm or renal colic due to its putative anticholinergic effects. These were later discovered to be all myths, as it carries an equal risk of addiction, possesses no advantageous effects on biliary spasm or renal colic compared to other opioids, and due to its toxic metabolite norpethidine is more toxic than other opioids - especially during long-term use. The norpethidine metabolite was found to have serotonergic effects, so pethidine could, unlike most opioids, contribute to serotonin syndrome.
Pethidine can be produced in a two-step synthesis. The first step is starting from benzyl cyanide and bis (chloroethyl) methylamine in the presence of sodium amide cyclization to piperidine. After the formation of the ester of the nitrile function.
...
Wikipedia
