Casodex
 |
|
 |
|
| Clinical data | |
|---|---|
| Pronunciation | Bicalutamide: /baɪkəˈluːtəmaɪd/ bye-kə-LOO-tə-myde Casodex: /ˈkeɪsoʊdɛks/ KAY-soh-deks |
| Trade names | Casodex, Cosudex, Calutide, Calumid, Kalumid, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697047 |
| License data |
|
| Pregnancy category |
|
| Routes of administration |
By mouth |
| Drug class | Non-steroidal antiandrogen |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Well-absorbed; absolute bioavailability unknown |
| Protein binding |
Racemate: 96.1% (R)-Isomer: 99.6% (Mainly to albumin) |
| Metabolism |
Liver (extensively): • Hydroxylation (CYP3A4) • Glucuronidation (UGT1A9) |
| Metabolites | • Bicalutamide glucuronide • Hydroxybicalutamide • Hydroxybicalutamide gluc. (All inactive) |
| Biological half-life | Acute: 5.8 days Chronic: 7–10 days |
| Excretion |
Feces: 43% Urine: 34% |
| Identifiers | |
|
|
| Synonyms | ICI-176,334 |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| ECHA InfoCard | 100.126.100 |
| Chemical and physical data | |
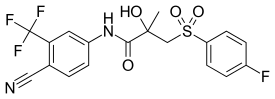
| Formula | C18H14F4N2O4S |
| Molar mass | 430.373 g/mol |
| 3D model (Jmol) | |
| Chirality | Racemic mixture |
| Melting point | 191 to 193 °C (376 to 379 °F) |
| Solubility in water | 0.005 mg/mL (20 °C) |
|
|
|
|
|
|
|
Bicalutamide, sold under the brand name Casodex among others, is an antiandrogen medication that is primarily used to treat prostate cancer. It is typically used together with a gonadotropin-releasing hormone (GnRH) analogue or surgical removal of the testicles to treat metastatic prostate cancer. Bicalutamide may also be used to treat excessive hair growth in women, as a component of hormone therapy for transgender women, to prevent priapism, and to treat early puberty in boys. It is taken by mouth.
Common side effects in men include breast enlargement, breast tenderness, and hot flashes. Other side effects in men include feminization and sexual dysfunction. While the medication appears to produce few side effects in women, its use in women is not recommended by the Food and Drug Administration. Use during pregnancy may harm the baby. Bicalutamide causes elevated liver enzymes in around 1% of people. It has been associated with rare cases of liver damage and lung toxicity. Although the risk of liver damage is small, monitoring of liver enzymes is recommended during treatment.
Bicalutamide is a member of the non-steroidal antiandrogen (NSAA) group of medications. It works by blocking the androgen receptor (AR), the biological target of the androgen sex hormones, such as testosterone and dihydrotestosterone (DHT). It does not lower androgen levels. Bicalutamide is well-absorbed, and its absorption is not affected by food. The terminal half-life of the medication is about six days. It is believed to cross the blood–brain barrier and have an effect on both the body and brain.
...
Wikipedia
