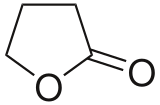
Butyrolactone
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Dihydrofuran-2(3H)-one
|
|
| Other names
GBL, butyrolactone, 1,4-lactone, 4-butyrolactone, 4-hydroxybutyric acid lactone, gamma-hydroxybutyric acid lactone, and oxolan-2-one
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.002.282 |
| KEGG | |
|
PubChem CID
|
|
| RTECS number | LU3500000 |
| UNII | |
|
|
|
|
| Properties | |
| C4H6O2 | |
| Molar mass | 86.09 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.1286 g/mL (15 °C), 1.1296 g/mL (20 °C) |
| Melting point | −43.53 °C (−46.35 °F; 229.62 K) |
| Boiling point | 204 °C (399 °F; 477 K) |
| Miscible | |
| Solubility | soluble in CCl4, methanol, ethanol, acetone, benzene, ethyl ether |
| Acidity (pKa) | 4.5 |
|
Refractive index (nD)
|
1.435, 1.4341 (20 °C) |
| Viscosity | 1.7 cp (25 °C) |
| Hazards | |
| Main hazards | Harmful |
| R-phrases | R22 R36 |
| S-phrases | S26 S36 |
| Flash point | 98 °C (208 °F; 371 K) (closed cup) |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
17.2 mL/kg (orally, rat) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
γ-Butyrolactone (GBL) is a hygroscopic colorless liquid with a weak characteristic odor. Soluble in water, GBL is a common solvent and reagent in chemistry as well as being used as a flavouring, as a cleaning solvent, as a superglue remover, and as a solvent in some wet aluminium electrolytic capacitors. In humans it acts as a prodrug for γ-hydroxybutyric acid (GHB), and it is used as a recreational intoxicant with effects similar to alcohol.
GBL has been found in extracts from samples of unadulterated wines. This finding indicates that GBL is a naturally occurring component in some wines and may be present in similar products. The concentration detected was approximately 5 μg/mL and was easily observed using a simple extraction technique followed by GC/MS analysis. GBL can be found in cheese flavourings but typically results in a content of 0.0002% GBL in the final foodstuff.
GBL is produced industrially by dehydrogenation of 1,4-butanediol. This route proceeds via dehydration of GHB.
In the laboratory, it may also be obtained via the oxidation of tetrahydrofuran (THF), for example with aqueous sodium bromate.
As a lactone, GBL is hydrolyzed under basic conditions, for example in a sodium hydroxide solution into sodium gamma-hydroxybutyrate, the sodium salt of gamma-hydroxybutyric acid. In acidic water, a mixture of the lactone and acid forms coexist in an equilibrium. These compounds then may go on to form the polymer poly(4-hydroxybutyrate). When treated with a non-nucleophilic base, such as lithium diisopropylamide, GBL undergoes deprotonation alpha to the carbonyl. The related compound caprolactone can be used to make a polyester in this manner.
...
Wikipedia
