Arsenic pentafluoride
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
Arsenic pentafluoride
|
|||
| Other names
Arsenic(V) fluoride,
Arsorane, pentafluoro- |
|||
| Identifiers | |||
|
7784-36-3 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChEBI |
CHEBI:30530 |
||
| ChemSpider |
74203 |
||
| ECHA InfoCard | 100.029.146 | ||
| PubChem | 82223 | ||
|
|||
|
|||
| Properties | |||
| AsF5 | |||
| Molar mass | 169.9136 g mol−1 | ||
| Appearance | colorless gas | ||
| Density | 2.138 kg/m3 (g/L) | ||
| Melting point | -79.8 ˚C | ||
| Boiling point | -52.8 ˚C | ||
| Solubility | Ethanol, Dimethylether, Benzene | ||
| Hazards | |||
|
EU classification (DSD)
|
Toxic (T) Dangerous for the environment (N) |
||
| R-phrases | R23/25, R50/53 | ||
| S-phrases | (S1/2), S20/21, S28, S45, S60, S61 | ||
| NFPA 704 | |||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
[1910.1018] TWA 0.010 mg/m3 | ||
|
REL (Recommended)
|
Ca C 0.002 mg/m3 [15-minute] | ||
|
IDLH (Immediate danger)
|
Ca [5 mg/m3 (as As)] | ||
| Related compounds | |||
|
Related group 5 fluorides
|
Phosphorus pentafluoride Antimony pentafluoride Bismuth pentafluoride |
||
|
Related compounds
|
Arsenic pentachloride Arsenic trifluoride Arsenic pentoxide |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Arsenic pentafluoride is a chemical compound of arsenic and fluorine. The oxidation state of arsenic is +5.
Arsenic pentafluoride can be prepared by direct combination of arsenic and fluorine:
It can also be prepared by the reaction of arsenic trifluoride and fluorine:
or the addition of fluorine to arsenic pentoxide or arsenic trioxide.
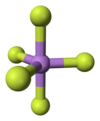
Arsenic pentafluoride is a colourless gas and has a trigonal bipyramidal structure. In the solid state the axial As-F bond lengths are 171.9 pm and the equatorial 166.8 pm.
Arsenic pentafluoride forms halide complexes and is a powerful acceptor as shown by the reaction with sulfur tetrafluoride forming an ionic complex.
Arsenic pentafluoride is an extremely dangerous toxin, mainly poisoning liver cells. It has a smell that is similar to vinyl chloride gas.
...
Wikipedia