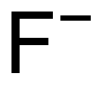Fluoride
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
Fluoride
|
|||
| Identifiers | |||
|
16984-48-8 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChEBI | CHEBI:17051 | ||
| ChEMBL |
ChEMBL1362 |
||
| ChemSpider |
26214 |
||
| 14905 | |||
| KEGG |
C00742 |
||
| MeSH | Fluoride | ||
| PubChem | 28179 | ||
|
|||
|
|||
| Properties | |||
| F− |
|||
| Molar mass | 19.00 g·mol−1 | ||
| Thermochemistry | |||
|
Std molar
entropy (S |
145.58 J/mol K (gaseous) | ||
|
Std enthalpy of
formation (ΔfH |
−333 kJ mol−1 | ||
| Related compounds | |||
|
Other anions
|
|||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
| Infobox references | |||
Fluoride /ˈflʊəraɪd/,/ˈflɔːraɪd/ is an inorganic, monatomic anion of fluorine with the chemical formula F−
. Fluoride is the simplest anion of fluorine. Its salts and minerals are important chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. In terms of charge and size, the fluoride ion resembles the hydroxide ion. Fluoride ions occur on earth in several minerals, particularly fluorite, but are only present in trace quantities in water. Fluoride contributes a distinctive bitter taste. It contributes no color to fluoride salts.
The systematic name fluoride, the valid IUPAC name, is determined according to the additive nomenclature. However, the name fluoride is also used in compositional IUPAC nomenclature which does not take the nature of bonding involved into account. Examples of such naming are sulfur hexafluoride and beryllium fluoride, which contain no fluoride ions whatsoever, although they do contain fluorine atoms.
...
Wikipedia