Ammonium cyanide
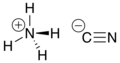 |
|||
|
|
|||
| Identifiers | |||
|---|---|---|---|
|
3D model (Jmol)
|
|||
| ChemSpider | |||
|
PubChem CID
|
|||
|
|||
|
|||
| Properties | |||
| NH4CN | |||
| Molar mass | 44.0559 g/mol | ||
| Appearance | colourless crystalline solid | ||
| Density | 1.02 g/cm3 | ||
| Boiling point | 36 °C (97 °F; 309 K) | ||
| very soluble | |||
| Solubility | very soluble in alcohol | ||
| Related compounds | |||
|
Other anions
|
Ammonium hydroxide Ammonium azide Ammonium nitrate |
||
|
Other cations
|
Sodium cyanide Potassium cyanide |
||
|
Related compounds
|
Ammonia Hydrogen cyanide |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Ammonium cyanide is an unstable inorganic compound with the formula NH4CN.
Ammonium cyanide is generally used in organic synthesis. Being unstable, it is not shipped or sold commercially.
Ammonium cyanide is prepared in solution by bubbling hydrogen cyanide into aqueous ammonia at a low temperature
It may be prepared by the reaction of calcium cyanide and ammonium carbonate:
In dry state, ammonium cyanide is made by heating a mixture of potassium cyanide or potassium ferrocyanide with ammonium chloride and condensing the vapours into ammonium cyanide crystals:
Ammonium cyanide decomposes to ammonia and hydrogen cyanide, often forming a black polymer of hydrogen cyanide:
It undergoes double decomposition reactions in solution with a number of metal salts.
It reacts with glyoxal, producing glycine (aminoacetic acid):
Reactions with ketones yield aminonitriles, as in the first step of the Strecker amino acid synthesis:
The solid or its solution is highly toxic. Ingestion can cause death. Exposure to the solid can be harmful as it decomposes to highly toxic hydrogen cyanide and ammonia.
Elemental composition: H 9.15%, C 27.23%, N 63.55%.
Ammonium cyanide may be analyzed by heating the salt and trapping the decomposed products: hydrogen cyanide and ammonia in water at low temperatures. The aqueous solution is analyzed for cyanide ion by silver nitrate titrimetric method or an ion-selective electrode method, and ammonia is measured by titration or electrode technique.
...
Wikipedia


