Ammonium cyanate
 |
|||
|
|
|||
 |
|||
| Names | |||
|---|---|---|---|
| Pronunciation | urea /jəˈriːə/, carbamide /ˈkɑːrbəmaɪd/ | ||
|
Preferred IUPAC name
Urea
|
|||
|
Systematic IUPAC name
Carbonic diamide
|
|||
| Other names
Carbamide
Carbonyl diamide Carbonyldiamine Diaminomethanal Diaminomethanone |
|||
| Identifiers | |||
|
57-13-6 |
|||
| 3D model (Jmol) | Interactive image | ||
| 635724 | |||
| ChEBI |
CHEBI:16199 |
||
| ChEMBL |
ChEMBL985 |
||
| ChemSpider |
1143 |
||
| DrugBank |
DB03904 |
||
| ECHA InfoCard | 100.000.286 | ||
| E number | E927b (glazing agents, ...) | ||
| 1378 | |||
| 4539 | |||
| KEGG |
D00023 |
||
| PubChem | 1176 | ||
| RTECS number | YR6250000 | ||
| UNII |
8W8T17847W |
||
|
|||
|
|||
| Properties | |||
| CH4N2O | |||
| Molar mass | 60.06 g·mol−1 | ||
| Appearance | White solid | ||
| Density | 1.32 g/cm3 | ||
| Melting point | 133 to 135 °C (271 to 275 °F; 406 to 408 K) | ||
| 1079 g/L (20 °C) 1670 g/L (40 °C) 2510 g/L (60 °C) 4000 g/L (80 °C) |
|||
| Solubility | 500 g/L glycerol 50g/L ethanol |
||
| Basicity (pKb) | 13.9 | ||
| -33.4·10−6 cm3/mol | |||
| Structure | |||
| 4.56 D | |||
| ThermochemistryCRC Handbook | |||
|
Std enthalpy of
formation (ΔfH |
-79.634 kcal/mol | ||
|
Gibbs free energy (ΔfG˚)
|
-47.12 kcal/mol | ||
| Pharmacology | |||
| B05BC02 (WHO) D02AE01 (WHO) | |||
| Hazards | |||
| Safety data sheet | JT Baker | ||
| Flash point | Non-flammable | ||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
8500 mg/kg (oral, rat) | ||
| Related compounds | |||
|
Related ureas
|
Thiourea Hydroxycarbamide |
||
|
Related compounds
|
Carbamide peroxide Urea phosphate Acetone Carbonic acid Carbonyl fluoride |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
50g/L ethanol
~4 g/L acetonitrile
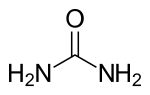
Urea, also known as carbamide, is an organic compound with the chemical formula CO(NH2)2. This amide has two –NH2 groups joined by a carbonyl (C=O) functional group.
Urea serves an important role in the metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance in the urine of mammals. It is a colorless, odorless solid, highly soluble in water, and practically non-toxic (LD50 is 15 g/kg for rats). Dissolved in water, it is neither acidic nor alkaline. The body uses it in many processes, most notably nitrogen excretion. The liver forms it by combining two ammonia molecules (NH3) with a carbon dioxide (CO2) molecule in the urea cycle. Urea is widely used in fertilizers as a source of nitrogen and is an important raw material for the chemical industry.
Friedrich Wöhler's discovery in 1828 that urea can be produced from inorganic starting materials was an important conceptual milestone in chemistry. It showed for the first time that a substance previously known only as a byproduct of life could be synthesized in the laboratory without biological starting materials, contradicting the widely held doctrine of vitalism.
...
Wikipedia