Carbamide peroxide
 |
|
 |
|
 |
|
| Names | |
|---|---|
| Other names
Urea peroxide, percarbamide, UHP
|
|
| Identifiers | |
|
124-43-6 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:75178 |
| ChemSpider |
29034 |
| ECHA InfoCard | 100.004.275 |
| PubChem | 31294 |
| UNII |
31PZ2VAU81 |
|
|
|
|
| Properties | |
| CH6N2O3 | |
| Molar mass | 94.07 g·mol−1 |
| Appearance | White solid |
| Melting point | 75 to 91.5 °C (167.0 to 196.7 °F; 348.1 to 364.6 K) (decomposes) |
| Pharmacology | |
| D02AE01 (WHO) | |
| Hazards | |
| Safety data sheet | External MSDS |
|
EU classification (DSD)
|
Explosive, |
| Flash point | 60 °C (140 °F; 333 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
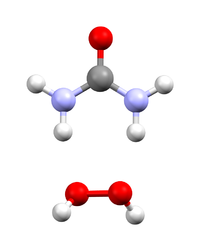
Hydrogen peroxide - urea is a solid composed of equal amounts of hydrogen peroxide and urea. This compound is a white crystalline solid, which dissolves in water to give free hydrogen peroxide. Often called carbamide peroxide in the dental applications, it is used as a source of hydrogen peroxide for bleaching, disinfection, and oxidation. Hydrogen peroxide - urea contains solid and water-free hydrogen peroxide, which offers a higher stability and better controllability than liquid hydrogen peroxide when used as oxidizing agent.
For the preparation of the compound urea (which is stable to oxidizing agents such as hydrogen peroxide) is dissolved in 30% hydrogen peroxide (molar ratio 2:3) at temperatures below 60 °C. On cooling, hydrogen peroxide - urea precipitates in the form of small platelets.
Determination of the hydrogen peroxide content by titration with potassium permanganate solution gives a value of 35.4% which corresponds to 97.8% of the theoretical maximum value. The remaining impurity consists of urea.
The adduct can be stabilized by adding about 1% sodium pyrophosphate, sodium hexametaphosphate, dihydroxybutanedioic acid or EDTANa2, since these complex the catalytically active heavy metal ions.
Akin to water of crystallization, hydrogen peroxide cocrystallizes with urea with the stoichiometry of 1:1. The compound is simply produced (on a scale of several hundred tonnes a year) by the dissolution of urea in excess concentrated hydrogen peroxide solution, followed by crystallization. The laboratory synthesis is analogous.
...
Wikipedia

