Acetic anhydrides
 |
|
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
Acetic anhydride
|
|
|
Systematic IUPAC name
Ethanoic anhydride
|
|
| Other names
Ethanoyl ethanoate
Acetic acid anhydride Acetyl acetate Acetyl oxide Acetic oxide |
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.241 |
| EC Number | 203-564-8 |
|
PubChem CID
|
|
| RTECS number | AK1925000 |
| UNII | |
|
|
|
|
| Properties | |
| C4H6O3 | |
| Molar mass | 102.09 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.082 g cm−3, liquid |
| Melting point | −73.1 °C (−99.6 °F; 200.1 K) |
| Boiling point | 139.8 °C (283.6 °F; 412.9 K) |
| 2.6 g/100 mL, see text | |
| Vapor pressure | 4 mmHg (20 °C) |
| -52.8·10−6 cm3/mol | |
|
Refractive index (nD)
|
1.3901 |
| Pharmacology | |
| Legal status |
|
| Hazards | |
| Safety data sheet | ICSC 0209 |
|
EU classification (DSD) (outdated)
|
Corrosive (C) |
| R-phrases (outdated) | R10, R20/22, R34 |
| S-phrases (outdated) | (S1/2), S26, S36/37/39, S45 |
| NFPA 704 | |
| Flash point | 49 °C (120 °F; 322 K) |
| 316 °C (601 °F; 589 K) | |
| Explosive limits | 2.7–10.3% |
| Lethal dose or concentration (LD, LC): | |
|
LC50 (median concentration)
|
1000 ppm (rat, 4 hr) |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
TWA 5 ppm (20 mg/m3) |
|
REL (Recommended)
|
C 5 ppm (20 mg/m3) |
|
IDLH (Immediate danger)
|
200 ppm |
| Related compounds | |
|
Related acid anhydrides
|
Propionic anhydride |
|
Related compounds
|
Acetic acid Acetyl chloride |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
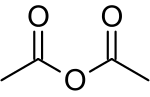
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a colorless liquid that smells strongly of acetic acid, which is formed by its reaction with moisture in the air.
Acetic anhydride, like most acid anhydrides, is a flexible molecule with a nonplanar structure. The pi system linkage through the central oxygen offers very weak resonance stabilization compared to the dipole-dipole repulsion between the two carbonyl oxygens. The energy barriers to bond rotation between each of the optimal aplanar conformations are quite low.
Like most acid anhydrides, the carbonyl carbon of acetic anhydride has electrophilic character, as the leaving group is carboxylate. The internal asymmetry may contribute to acetic anhydride's potent electrophilicity as the asymmetric geometry makes one side of a carbonyl carbon more reactive than the other, and in doing so tends to consolidate the electropositivity of a carbonyl carbon to one side (see electron density diagram).
Acetic anhydride was first synthesized in 1852 by the French chemist Charles Frédéric Gerhardt (1816-1856) by heating potassium acetate with benzoyl chloride.
Acetic anhydride is produced by carbonylation of methyl acetate:
The Tennessee Eastman acetic anhydride process involves the conversion of methyl acetate to methyl iodide and an acetate salt. Carbonylation of the methyl iodide in turn affords acetyl iodide, which reacts with acetate salts or acetic acid to give the product. Rhodium chloride in the presence of lithium iodide is employed as catalysts. Because acetic anhydride is not stable in water, the conversion is conducted under anhydrous conditions.
...
Wikipedia

