Zonisamide
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Zonegran |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603008 |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~100% |
| Protein binding | 40% |
| Metabolism | Hepatic through CYP3A4 |
| Biological half-life | 63 hours in plasma |
| Excretion | Renal (62%); Faeces (3%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| ECHA InfoCard | 100.118.526 |
| Chemical and physical data | |
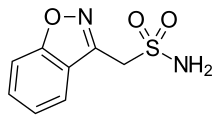
| Formula | C8H8N2O3S |
| Molar mass | 212.227 g/mol |
| 3D model (Jmol) | |
| Melting point | 162 °C (324 °F) |
|
|
|
|
Zonisamide is a sulfonamide anticonvulsant approved for use as an therapy in adults with partial-onset seizures; infantile spasm, mixed seizure types of Lennox–Gastaut syndrome, myoclonic, and generalized tonic clonic seizure.
Zonisamide is approved in the United States, United Kingdom, and Australia for adjunctive treatment of partial seizures in adults and in Japan for both adjunctive and monotherapy for partial seizures (simple, complex, secondarily generalized), generalized (tonic, tonic-clonic (grand mal), and atypical absence) and combined seizures. For epilepsy, most studies have used oral zonisamide in daily doses ranging from 200 to 600 milligrams/day, divided in 2 daily doses, adjusted to maintain serum levels of 15 to 40 micrograms/milliliter
In an open-label trial zonisamide attenuated the symptoms of tardive dyskinesia.
It has also been studied for obesity with significant positive effects on body weight and there are three ongoing clinical trials for this indication. It is to be sold, when combined with bupropion, under the brand name Empatic.
Zonisamide has been studied for and used as a migraine preventative medication, and has also been shown to be effective in some cases of neuropathic pain.
It has also been used off-label by psychiatrists as a mood stabilizer to treat bipolar depression.
Adverse effects by incidence:
Very common (>10% incidence) adverse effects include:
Common (1-10% incidence) adverse effects include:
Zonisamide and other carbonic anhydrase inhibitors such as topiramate, furosemide, and hydrochlorothiazide have been known to interfere with amobarbital, which has led to inadequate anesthetization during the Wada test. Zonisamide may also interact with other carbonic anhydrase inhibitors to increase the potential for metabolic acidosis.
...
Wikipedia
