Triphenylstibine
 |
|
| Names | |
|---|---|
|
IUPAC name
Triphenylstibine
|
|
| Other names
Triphenylantimony
|
|
| Identifiers | |
|
603-36-1 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
11284 |
| ECHA InfoCard | 100.009.125 |
| PubChem | 11777 |
| RTECS number | WJ1400000 |
|
|
|
|
| Properties | |
| C18H15Sb | |
| Molar mass | 353.07 g/mol |
| Appearance | Colourless solid |
| Density | 1.53 g/cm3 |
| Melting point | 52 to 54 °C (126 to 129 °F; 325 to 327 K) |
| Boiling point | 377 °C (711 °F; 650 K) |
| insoluble | |
| Structure | |
| trigonal pyramidal | |
| Hazards | |
| Main hazards | mildly toxic |
| R-phrases | 20/22-51/53 |
| S-phrases | 61 |
| NFPA 704 | |
| Related compounds | |
|
Related compounds
|
Triphenylphosphine Triphenylarsine Stibine |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
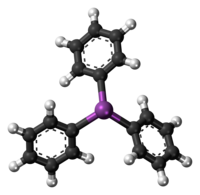
Triphenylstibine is the chemical compound with the formula Sb(C6H5)3. Abbreviated SbPh3, this colourless solid is often considered the prototypical organoantimony compound. It is used as a ligand in coordination chemistry and as a reagent in organic synthesis.
Like the related molecules triphenylphosphine and triphenylarsine, SbPh3 is pyramidal with a propeller-like arrangement of the phenyl groups. The Sb-C distances average 2.14-2.17 Å and the C-Sb-C angle are 95°.
SbPh3 was first reported in 1886, being prepared from antimony trichloride by the reaction:
The modern method employs the Grignard reaction, using phenylmagnesium bromide and SbCl3.
...
Wikipedia

