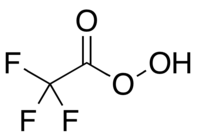
Trifluoroperacetic acid
 |
|
| Names | |
|---|---|
|
IUPAC name
2,2,2-trifluoroethaneperoxoic acid
|
|
Other names
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C2HF3O3 | |
| Molar mass | 130.02 g·mol−1 |
| Boiling point | 162 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Trifluoroperacetic acid (trifluoroperoxyacetic acid, TFPAA) is the peroxy acid analog of trifluoroacetic acid and has the condensed structural formula CF
3COOOH. It is a strong oxidizing agent for organic oxidation reactions, such as in Baeyer–Villiger oxidations of ketones. It is the most reactive of the organic peroxy acids, allowing it to successfully oxidise relatively unreactive alkenes to epoxides where other peroxy acids are ineffective. It can also oxidise the chalcogens in some functional groups, such as by transforming selenoethers to selenones. It is a potentially explosive material and is not commercially available, but can be quickly prepared immediately prior to use when needed. Its use as a laboratory reagent was pioneered and developed by William D. Emmons.
At standard ambient temperature and pressure, trifluoroperacetic acid is a colourless liquid with a boiling point of 162 °C. It is soluble in acetonitrile, dichloromethane, diethyl ether, and sulfolane, and readily reacts with water. Like all peroxy acids, it is potentially explosive and requires careful handling. It is not commercially available, but can be stored for up to several weeks at −20 °C. Some preparative methods result in mixtures containing residual hydrogen peroxide and trifluoroacetic acid, and heating such a mixture is extremely hazardous; the hydrogen peroxide can be decomposed using manganese dioxide for safety prior to heating.
...
Wikipedia
