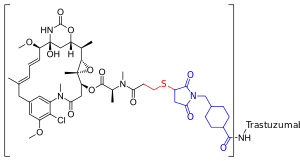
Trastuzumab emtansine
 |
|
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized (from mouse) |
| Clinical data | |
| Trade names | Kadcyla, Kadccyla |
| Pregnancy category |
|
| Routes of administration |
Intravenous infusion |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | N/A |
| Protein binding | 93% (in vitro) |
| Metabolism | Hepatic (CYP3A4/3A5-mediated) |
| Biological half-life | 4 days |
| Identifiers | |
| CAS Number | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C6448H9948N1720O2012S44·(C47H62ClN4O13S)n |
| Molar mass | 148.5 kg/mol |
|
|
|
Trastuzumab emtansine also known as ado-trastuzumab emtansine and sold under the trade name Kadcyla, is an antibody-drug conjugate consisting of the monoclonal antibody trastuzumab (Herceptin) linked to the cytotoxic agent emtansine (DM1). Trastuzumab alone stops growth of cancer cells by binding to the HER2/neu receptor, whereas DM1 enters cells and destroys them by binding to tubulin. Trastuzumab binding to Her2 prevents homodimerization or heterodimerization (Her2/Her3) of the receptor, ultimately inhibiting the activation of MAPK and PI3K/Akt cellular signalling pathways. Because the monoclonal antibody targets HER2, and HER2 is only over-expressed in cancer cells, the conjugate delivers the toxin specifically to tumor cells. The conjugate is abbreviated T-DM1.
In the EMILIA clinical trial of women with advanced HER2 positive breast cancer who were already resistant to trastuzumab alone, it improved median overall survival by 5.8 months (30.9 months vs. 25.1 months) compared to the combination of lapatinib and capecitabine. Based on that trial, the U.S. Food and Drug Administration (FDA) approved marketing on February 22, 2013.
Trastuzumab emtansine was developed by Genentech, a subsidiary group of Roche, and is manufactured by Lonza. The planned cost is expected to be $9,800 a month, or $94,000 for a typical course of treatment.
In the United States, ado-trastuzumab emtansine was approved specifically for treatment of HER2-positive metastatic breast cancer (mBC) in patients who have been treated previously with trastuzumab and a taxane (paclitaxel or docetaxel), and who have already been treated for mBC or developed tumor within six months of adjuvant therapy.
...
Wikipedia
