Tibolone
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration |
oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
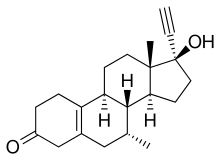
| Synonyms | 7α-Methylnoretynodrel; Org OD 14 (7α,17β)-17-ethynyl-17-hydroxy-7-methylestr-5(10)-en-3-one |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.024.609 |
| Chemical and physical data | |
| Formula | C21H28O2 |
| Molar mass | 312.446 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Tibolone (INN, USAN, BAN) (brand name Livial, Tibofem), also known as 7α-methylnoretynodrel, is a synthetic steroid drug with estrogenic, progestogenic, and weak androgenic actions which was introduced in 1988 and is used widely in Europe, Asia, Australasia, and, with the exception of the United States (where it is not available), the rest of the world. It is used mainly for treatment of endometriosis, as well as hormone replacement therapy in post-menopausal women. Tibolone has similar or greater efficacy compared to older hormone replacement drugs, but shares a similar side effect profile. It has also been investigated as a possible treatment for female sexual dysfunction.
Tibolone is a 19-nortestosterone derivative and is related structurally to other 19-nortestosterone progestins. It is the 7α-methyl derivative of noretynodrel.
Tibolone possesses a complex pharmacology. Its two major active metabolites, 3α-hydroxytibolone and 3β-hydroxytibolone, act as potent, fully activating agonists of the estrogen receptor (ER), with a high preference for ERα. Tibolone and its metabolite Δ4-tibolone act as agonists of the progesterone and androgen receptors, while 3α-hydroxytibolone and 3β-hydroxytibolone, conversely, act as antagonists of these receptors. Lastly, tibolone, 3α-hydroxytibolone, and 3β-hydroxytibolone act as antagonists of the glucocorticoid and mineralocorticoid receptors, with preference for the mineralocorticoid receptor.
...
Wikipedia
