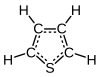
Thiophene
|
|
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Thiophene
|
|||
| Other names
Thiofuran
Thiacyclopentadiene Thiole |
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.392 | ||
|
PubChem CID
|
|||
| RTECS number | XM7350000 | ||
| UNII | |||
|
|||
|
|||
| Properties | |||
| C4H4S | |||
| Molar mass | 84.14 g/mol | ||
| Appearance | colorless liquid | ||
| Density | 1.051 g/mL, liquid | ||
| Melting point | −38 °C (−36 °F; 235 K) | ||
| Boiling point | 84 °C (183 °F; 357 K) | ||
| -57.38·10−6 cm3/mol | |||
|
Refractive index (nD)
|
1.5287 | ||
| Viscosity | 0.8712 cP at 0.2 °C 0.6432 cP at 22.4 °C |
||
| Hazards | |||
| Safety data sheet | External MSDS, External MSDS | ||
|
EU classification (DSD)
|
not listed | ||
| NFPA 704 | |||
| Flash point | −1 °C (30 °F; 272 K) | ||
| Related compounds | |||
|
Related thioethers
|
Tetrahydrothiophene Diethyl sulfide |
||
|
Related compounds
|
Furan Pyrrole |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Thiophene, also commonly called thiofuran, is a heterocyclic compound with the formula C4H4S. Consisting of a flat five-membered ring, it is aromatic as indicated by its extensive substitution reactions. Related to thiophene are benzothiophene and dibenzothiophene, containing the thiophene ring fused with one and two benzene rings, respectively. Compounds analogous to thiophene include furan (C4H4O) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring.
Thiophene was discovered as a contaminant in benzene. It was observed that isatin (an indole) forms a blue dye if it is mixed with sulfuric acid and crude benzene. The formation of the blue indophenin had long been believed to be a reaction of benzene itself. Viktor Meyer was able to isolate thiophene as the actual substance responsible for this reaction.
Thiophene and its derivatives occur in petroleum, sometimes in concentrations up to 1–3%. The thiophenic content of oil and coal is removed via the hydrodesulfurization (HDS) process. In HDS, the liquid or gaseous feed is passed over a form of molybdenum disulfide catalyst under a pressure of H2. Thiophenes undergo hydrogenolysis to form hydrocarbons and hydrogen sulfide. Thus, thiophene itself is converted to butane and H2S. More prevalent and more problematic in petroleum are benzothiophene and dibenzothiophene.
...
Wikipedia