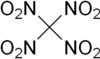Tetranitromethane
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
Tetranitromethane
|
|||
| Other names
TNM
Tetan |
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.007.359 | ||
| KEGG | |||
| RTECS number | PB4025000 | ||
| UN number | 1510 | ||
|
|||
|
|||
| Properties | |||
| CN4O8 | |||
| Molar mass | 196.04 g/mol | ||
| Appearance | Colorless to pale-yellow liquid or solid | ||
| Odor | Pungent | ||
| Density | 1.623 g/cm3 | ||
| Melting point | 13.8 °C (56.8 °F; 286.9 K) | ||
| Boiling point | 126 °C (259 °F; 399 K) | ||
| insol | |||
| Vapor pressure | 8 mmHg (20°C) | ||
| -43.02·10−6 cm3/mol | |||
| Hazards | |||
| Main hazards | Oxidant, can form explosive mixtures | ||
| Safety data sheet | ICSC 1468 | ||
|
EU classification (DSD)
|
 
|
||
| R-phrases | R8 R23/24/25 R36/38 R45 | ||
| S-phrases | S17 S45 | ||
| NFPA 704 | |||
| Lethal dose or concentration (LD, LC): | |||
|
LC50 (median concentration)
|
18 ppm (rat, 4 hr) 100 ppm (cat, 20 min) 54 ppm (mouse, 4 hr) |
||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
TWA 1 ppm (8 mg/m3) | ||
|
REL (Recommended)
|
TWA 1 ppm (8 mg/m3) | ||
|
IDLH (Immediate danger)
|
4 ppm | ||
| Related compounds | |||
|
Related compounds
|
Hexanitroethane Octanitropentane Trinitromethane |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Tetranitromethane or TNM is an organic oxidizer with chemical formula C(NO2)4. Its chemical structure consists of four nitro groups attached to one carbon atom. In 1857 it was first synthesised by the reaction of sodium cyanoacetamide with nitric acid.
It has been investigated for use as an oxidizer in bipropellant rockets; however, its high freezing temperature makes it unsuitable. Highly purified tetranitromethane cannot be made to explode, but its sensitivity is increased dramatically by oxidizable contaminants, such as anti-freezing additives. This makes it effectively unusable as a propellant. In the laboratory it is used as a reagent for the detection of double bonds in organic compounds and as a nitrating reagent. It has also found use as an additive to diesel fuel to increase the cetane number.
TNM is a pale yellow liquid that can be prepared in the laboratory by the nitration of acetic anhydride with anhydrous nitric acid (Chattaway's method). This method was attempted on an industrial scale in the 1950s by Nitroform Products Company in Newark, USA, but the entire plant was destroyed by an explosion in 1953.
The first industrial scale production was started in Germany during World War II in an effort to improve the cetane number of diesel fuel. This process improved the original method, which started with acetic acid and nitric acid. Without regard to yield or cost, approximately 10 tons of TNM were produced in a few weeks. However, this production process has not been used again industrially after the end of the war, because of high associated costs.
For commercial use a cheaper method starting from acetylene has been used. First, nitric acid containing mercuric nitrate is reduced by acetylene, resulting in nitroform (trinitromethane) and a mixture of carbon dioxide and nitrogen oxide as waste gas. The nitrogen oxides are valuable and normally recovered as nitric acid in an absorption tower. The resulting nitroform is converted to TNM by adding nitric and sulfuric acid at higher temperatures. With this method a yield of 90% (based on nitric acid) before purification can be reached.
...
Wikipedia