Tamoxifen
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Nolvadex, Genox, Tamifen, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682414 |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 99% |
| Metabolism | Hepatic (CYP3A4, 2C9 and 2D6) |
| Biological half-life | 5-7 days |
| Excretion | Faeces (65%), urine (9%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.031.004 |
| Chemical and physical data | |
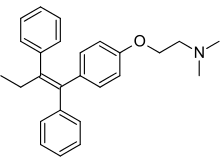
| Formula | C26H29NO |
| Molar mass | 371.515 g/mol 563.638 g/mol (citrate salt) |
| 3D model (Jmol) | |
|
|
|
|
Tamoxifen (TMX), sold under the brand name Nolvadex among others, is a medication that is used to prevent breast cancer in women and treat breast cancer in women and men. It is also being studied for other types of cancer. It has been used for Albright syndrome. Tamoxifen is typically taken daily by mouth for five years for breast cancer.
Serious side effects include a small increased risk of uterine cancer, stroke, vision problems, and pulmonary embolism. Common side effects include irregular periods, weight loss, and hot flashes. It may cause harm to the baby if taken during pregnancy or breastfeeding. It is a selective estrogen-receptor modulator (SERM) that works both by decreasing factors that increase the growth of breast cells and increasing factors that decrease the growth of breast cells. It is of the triphenylethylene group.
Tamoxifen was discovered in 1967. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. Tamoxifen is available as a generic medication. The wholesale price in the developing world is about 0.07 to 0.23 USD per day. In the United States it costs about 2 USD a day.
Tamoxifen is currently used for the treatment of both early and advanced estrogen receptor (ER)-positive (ER+) breast cancer in pre- and post-menopausal women. Additionally, it is the most common hormone treatment for male breast cancer. It is also approved by the FDA for the prevention of breast cancer in women at high risk of developing the disease. It has been further approved for the reduction of contralateral (in the opposite breast) cancer. The use of tamoxifen is recommended for 10 years.
...
Wikipedia
