Sulforaphane
 |
|
 |
|
 |
|
| Names | |
|---|---|
|
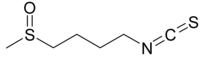
IUPAC name
1-Isothiocyanato-4-methylsulfinylbutane
|
|
| Identifiers | |
|
4478-93-7 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:47807 |
| ChEMBL |
ChEMBL48802 |
| ChemSpider |
5157 |
| PubChem | 5350 |
|
|
|
|
| Properties | |
| C6H11NOS2 | |
| Molar mass | 177.29 g/mol |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Sulforaphane is a compound within the isothiocyanate group of organosulfur compounds. It is obtained from cruciferous vegetables such as broccoli, Brussels sprouts or cabbages. It is produced when the enzyme myrosinase transforms glucoraphanin, a glucosinolate, into sulforaphane upon damage to the plant (such as from chewing), which allows the two compounds to mix and react. Young sprouts of broccoli and cauliflower are particularly rich in glucoraphanin.
Sulforaphane was identified in broccoli sprouts, which, of the cruciferous vegetables, have the highest concentration of sulforaphane. It is also found in Brussels sprout, cabbage, cauliflower, bok choy, kale, collards, Chinese broccoli, broccoli raab, kohlrabi, mustard, turnip, radish, arugula, and watercress.
Although there is basic research on how sulforaphane may affect mechanisms in vivo, there is limited evidence to date for its effects on human diseases. While investigations support the effect of sulforaphane on Nrf2 activity, it has far less effect than synthetic triterpenoid analogues.
...
Wikipedia
