Squalene monooxygenase
| Squalene epoxidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
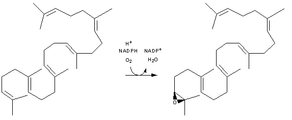
Chemical reaction catalyzed by squalene epoxidase.
|
|||||||||
| Identifiers | |||||||||
| EC number | 1.14.13.132 | ||||||||
| CAS number | 9029-62-3 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
|
|||||||||
| Search | |
|---|---|
| PMC | articles |
| PubMed | articles |
| NCBI | proteins |
Squalene monooxygenase (also called squalene epoxidase) is an enzyme that uses NADPH and molecular oxygen to oxidize squalene to 2,3-oxidosqualene (squalene epoxide). Squalene epoxidase catalyzes the first oxygenation step in sterol biosynthesis and is thought to be one of the rate-limiting enzymes in this pathway. In humans, squalene epoxidase is encoded by the SQLE gene. Squalene monooxygenase (SqMO) was formerly referred to as squalene epoxidase (SqE) in the literature.
Squalene monooxygenase is a flavoprotein monooxygenase. Flavoprotein monooxygenase form flavin hydroperoxides at the enzyme active site, which then transfer the terminal oxygen atom of the hydroperoxide to the substrate. Squalene monooxygenase differs from other flavin monooxygenases in that the oxygen is inserted as an epoxide rather than as a hydroxyl group. Squalene monooxygenase contains a loosely bound FAD flavin and obtains electrons from NADPH-, rather than binding the nicotinamide cofactor NADPH directly.
...
Wikipedia




