Prucalopride
 |
|
| Clinical data | |
|---|---|
| Trade names | Resolor, Resotran |
| AHFS/Drugs.com | International Drug Names |
| License data | |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
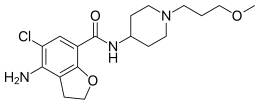
| Formula | C18H26ClN3O3 |
| Molar mass | 367.870 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Prucalopride (brand name Resolor, developed by Johnson & Johnson and licensed to Movetis) is a drug acting as a selective, high affinity 5-HT4 receptor agonist which targets the impaired motility associated with chronic constipation, thus normalizing bowel movements. Prucalopride was approved for use in Europe in 2009, in Canada (named Resotran) on December 7, 2011 and in Israel in 2014 but it has not been approved by the Food and Drug Administration for use in the United States. The drug has also been tested for the treatment of chronic intestinal pseudo-obstruction.
Prucalopride, a first in class dihydro-benzofuran-carboxamide, is a selective, high affinity serotonin (5-HT4) receptor agonist with enterokinetic activities. Prucalopride alters colonic motility patterns via serotonin 5-HT4 receptor stimulation: it stimulates colonic mass movements, which provide the main propulsive force for defecation.
The observed effects are exerted via highly selective action on 5-HT4 receptors: prucalopride has >150-fold higher affinity for 5-HT4 receptors than for other receptors. Prucalopride differs from other 5-HT4 agonists such as tegaserod and cisapride, which at therapeutic concentrations also interact with other receptors (5-HT1B/D and the cardiac human ether-a-go-go K+ or hERG channel respectively) and this may account for the adverse cardiovascular events that have resulted in the restricted availability of these drugs.Clinical trials evaluating the effect of prucalopride on QT interval and related adverse events have not demonstrated significant differences compared with placebo.
...
Wikipedia
