Phosphothreonine
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
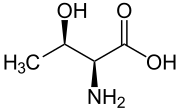
Threonine
|
|
| Other names
2-Amino-3-hydroxybutanoic acid
|
|
| Identifiers | |
|
80-68-2 72-19-5 (L-isomer) |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:57926 |
| ChEMBL |
ChEMBL291747 |
| ChemSpider |
6051 |
| DrugBank |
DB00156 |
| ECHA InfoCard | 100.000.704 |
| EC Number | 201-300-6 |
| 4785 | |
| PubChem | 6288 |
| UNII |
2ZD004190S |
|
|
|
|
| Properties | |
| C4H9NO3 | |
| Molar mass | 119.12 g·mol−1 |
| (H2O, g/dl) 10.6(30°),14.1(52°),19.0(61°) | |
| Acidity (pKa) | 2.63 (carboxyl), 10.43 (amino) |
| Supplementary data page | |
|
Refractive index (n), Dielectric constant (εr), etc. |
|
|
Thermodynamic
data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Threonine (abbreviated as Thr or T) encoded by the codons ACU, ACC, ACA, and ACG is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+
3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and an alcohol containing side chain, classifying it as a polar, uncharged (at physiological pH) amino acid. It is essential in humans, meaning the body cannot synthesize it, and must be ingested in our diet. Threonine is synthesized from aspartate in bacteria such as E. coli.
Threonine sidechains are often hydrogen bonded; the commonest small motifs formed are ST turns, ST motifs (often at the beginning of alpha helices) and ST staples (usually at the middle of alpha helices).
The threonine residue is susceptible to numerous posttranslational modifications. The hydroxyl side-chain can undergo O-linked glycosylation. In addition, threonine residues undergo phosphorylation through the action of a threonine kinase. In its phosphorylated form, it can be referred to as phosphothreonine.
It is a precursor of glycine, and can be used as a prodrug to reliably elevate brain glycine levels.
...
Wikipedia
