Phenmetrazine
 |
|
| Clinical data | |
|---|---|
| Routes of administration |
Oral, Intravenous, Vaporized, Insufflated, Suppository |
| ATC code |
|
| Pharmacokinetic data | |
| Biological half-life | 8 hours |
| Excretion | Renal |
| Legal status | |
| Legal status |
|
| ECHA InfoCard | 100.004.677 |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
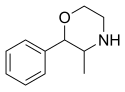
| Formula | C11H15NO |
| Molar mass | 177.2456 |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Phenmetrazine (INN, USAN, BAN) (brand name Preludin, and many others) is a stimulant drug that was previously used as an appetite suppressant, but has since been withdrawn from the market. It was initially replaced by its analogue phendimetrazine which functions as a prodrug to phenmetrazine, but now it is rarely prescribed, due to concerns of abuse and addiction. Chemically, phenmetrazine is a substituted amphetamine with a morpholine ring.
Phenmetrazine was first patented in Germany in 1952 by Boehringer-Ingelheim, with some pharmacological data published in 1954. It was the result of a search by Thomä and Wick for an anorectic drug without the side-effects of amphetamine. Phenmetrazine was introduced into clinical use in 1954 in Europe.
In clinical use, phenmetrazine produces less nervousness, hyperexcitability, euphoria and insomnia than drugs of the amphetamine family. It tends not to increase heart rate as much as other stimulants. Due to the relative lack of side effects, one study found it well tolerated in children. In a study of the effectiveness on weight loss between phenmetrazine and dextroamphetamine, phenmetrazine was found to be slightly more effective.
...
Wikipedia
