PDGF-R
| platelet-derived growth factor receptor, alpha polypeptide | |
|---|---|
| Identifiers | |
| Symbol | PDGFRA |
| Entrez | 5156 |
| HUGO | 8803 |
| OMIM | 173490 |
| RefSeq | NM_006206 |
| UniProt | P16234 |
| Other data | |
| Locus | Chr. 4 q12 |
| platelet-derived growth factor receptor, beta polypeptide | |
|---|---|
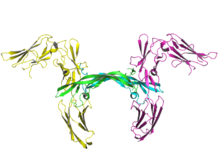
Ribbon image of two molecules of human PDGF receptor beta (yellow and magenta) in complex with dimeric PDGF-B (cyan and green).
|
|
| Identifiers | |
| Symbol | PDGFRB |
| Alt. symbols | PDGFR |
| Entrez | 5159 |
| HUGO | 8804 |
| OMIM | 173410 |
| RefSeq | NM_002609 |
| UniProt | P09619 |
| Other data | |
| Locus | Chr. 5 q31-q32 |
Platelet-derived growth factor receptors (PDGF-R) are cell surface tyrosine kinase receptors for members of the platelet-derived growth factor (PDGF) family. PDGF subunits -A and -B are important factors regulating cell proliferation, cellular differentiation, cell growth, development and many diseases including cancer. There are two forms of the PDGF-R, alpha and beta each encoded by a different gene. Depending on which growth factor is bound, PDGF-R homo- or heterodimerizes.
The PDGF family consists of PDGF-A, -B, -C and -D, which form either homo- or heterodimers (PDGF-AA, -AB, -BB, -CC, -DD). The four PDGFs are inactive in their monomeric forms. The PDGFs bind to the protein tyrosine kinase receptors PDGF receptor-α and -β. These two receptor isoforms dimerize upon binding the PDGF dimer, leading to three possible receptor combinations, namely -αα, -ββ and -αβ. The extracellular region of the receptor consists of five immunoglobulin-like domains while the intracellular part is a tyrosine kinase domain. The ligand-binding sites of the receptors are located to the three first immunoglobulin-like domains. PDGF-CC specifically interacts with PDGFR-αα and -αβ, but not with -ββ, and thereby resembles PDGF-AB. PDGF-DD binds to PDGFR-ββ with high affinity, and to PDGFR-αβ to a markedly lower extent and is therefore regarded as PDGFR-ββ specific. PDGF-AA binds only to PDGFR-αα, while PDGF-BB is the only PDGF that can bind all three receptor combinations with high affinity.
...
Wikipedia
