Octreotide
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Sandostatin |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration |
Subcutaneous, intramuscular, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 60% (IM), 100% (SC) |
| Protein binding | 40–65% |
| Metabolism | Hepatic |
| Biological half-life | 1.7–1.9 hours |
| Excretion | Urine (32%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
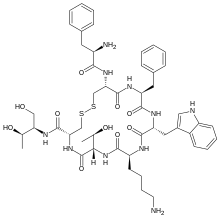
| Formula | C49H66N10O10S2 |
| Molar mass | 1019.24 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
| Identifiers | |
|---|---|
| Symbol | N/A |
| OPM superfamily | 167 |
| OPM protein | 1soc |
Octreotide (brand name Sandostatin,Novartis Pharmaceuticals) is an octapeptide that mimics natural somatostatin pharmacologically, though it is a more potent inhibitor of growth hormone, glucagon, and insulin than the natural hormone. It was first synthesized in 1979 by the chemist Wilfried Bauer.
Octreotide is used for the treatment of growth hormone producing tumors (acromegaly and gigantism), pituitary tumors that secrete thyroid stimulating hormone (thyrotropinoma), diarrhea and flushing episodes associated with carcinoid syndrome, and diarrhea in people with vasoactive intestinal peptide-secreting tumors (VIPomas).
Octreotide is often given as an infusion for management of acute haemorrhage from esophageal varices in liver cirrhosis on the basis that it reduces portal venous pressure, though current evidence suggests that this effect is transient and does not improve survival.
Octreotide is used in nuclear medicine imaging by labelling with indium-111 (Octreoscan) to noninvasively image neuroendocrine and other tumours expressing somatostatin receptors. More recently, it has been radiolabelled with carbon-11 as well as gallium-68, enabling imaging with positron emission tomography (PET), which provides higher resolution and sensitivity.
...
Wikipedia
