Neopentane
 |
|||
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
2,2-Dimethylpropane
|
|||
| Other names
neopentane (no longer recommended)
tetramethylmethane |
|||
| Identifiers | |||
|
463-82-1 |
|||
| 3D model (Jmol) | Interactive image | ||
| 1730722 | |||
| ChEBI |
CHEBI:30358 |
||
| ChemSpider |
9646 |
||
| ECHA InfoCard | 100.006.677 | ||
| EC Number | 207-343-7 | ||
| 1850 | |||
| MeSH | neopentane | ||
| PubChem | 10041 | ||
| UNII |
M863R1J0BP |
||
|
|||
|
|||
| Properties | |||
| C5H12 | |||
| Molar mass | 72.15 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Odor | Odorless | ||
| Density | 586 mg mL−1 | ||
| Melting point | −21 to −15 °C; −6 to 5 °F; 252 to 258 K | ||
| Boiling point | 9.0 to 10.0 °C; 48.1 to 49.9 °F; 282.1 to 283.1 K | ||
| Vapor pressure | 146 kPa (at 20 °C) | ||
|
Henry's law
constant (kH) |
4.7 nmol Pa−1 kg−1 | ||
| Thermochemistry | |||
| 121.07–120.57 J K−1 mol−1 | |||
|
Std molar
entropy (S |
217 J K−1 mol−1 | ||
|
Std enthalpy of
formation (ΔfH |
−168.5–−167.3 kJ mol−1 | ||
|
Std enthalpy of
combustion (ΔcH |
−3.51506–−3.51314 MJ mol−1 | ||
| Hazards | |||
|
EU classification (DSD)
|
|
||
| R-phrases | R12, R51/53 | ||
| S-phrases | (S2), S16, S33 | ||
| NFPA 704 | |||
| Related compounds | |||
|
Related alkanes
|
|||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
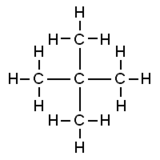
Neopentane, also called 2,2-dimethylpropane, is a double-branched-chain alkane with five carbon atoms. Neopentane is a flammable gas at room temperature and pressure which can condense into a highly volatile liquid on a cold day, in an ice bath, or when compressed to a higher pressure.
Neopentane is the simplest alkane with a quaternary carbon, and has achiral tetrahedral symmetry. It is one of the three structural isomers with the molecular formula C5H12 (pentanes), the other two being n-pentane and isopentane. Out of these three, it is the only one to be a gas at standard conditions; the other being liquids.
The traditional name neopentane was still retained in the 1993 IUPAC recommendations, but is no longer recommended according to the 2013 recommendations. The preferred IUPAC name is the systematic name 2,2-dimethylpropane, but the substituent numbers are superfluous because it is the only possible “dimethylpropane.”
A neopentyl substituent, often symbolized by "Np", has the structure Me3C-CH2- for instance neopentyl alcohol (Me3CCH2OH or NpOH). As Np also symbolises the element neptunium (atomic number 93) one should use this abbreviation with care.
The obsolete name tetramethylmethane is also used, especially in older sources.
The boiling point of neopentane is only 9.5 °C, significantly lower than those of isopentane (27.7 °C) and normal pentane (36.0 °C). Therefore, neopentane is a gas at room temperature and atmospheric pressure, while the other two isomers are (barely) liquids.
...
Wikipedia