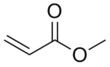
Methyl acrylate
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
Methyl prop-2-enoate
|
|||
| Other names
Methyl acrylate
Methyl propenoate Methoxycarbonylethylene Curithane 103 |
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.002.274 | ||
| KEGG | |||
|
PubChem CID
|
|||
|
|||
|
|||
| Properties | |||
| C4H6O2 | |||
| Molar mass | 86.09 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Acrid | ||
| Density | 0.95 g/cm3 | ||
| Melting point | −74 °C (−101 °F; 199 K) | ||
| Boiling point | 80 °C (176 °F; 353 K) | ||
| 5 g/100 mL | |||
| Vapor pressure | 65 mmHg (20°C) | ||
| Hazards | |||
| Main hazards | Harmful (Xn); Highly flammable (F+) | ||
| Safety data sheet | Oxford MSDS | ||
| Flash point | −3 °C (27 °F; 270 K) | ||
| Explosive limits | 2.8%-25% | ||
| Lethal dose or concentration (LD, LC): | |||
|
LC50 (median concentration)
|
3575 ppm (mouse) 1350 ppm (rat, 4 hr) 1000 ppm (rat, 4 hr) 2522 ppm (rabbit, 1 hr) |
||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
TWA 10 ppm (35 mg/m3) [skin] | ||
|
REL (Recommended)
|
TWA 10 ppm (35 mg/m3) [skin] | ||
|
IDLH (Immediate danger)
|
250 ppm | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Methyl acrylate is an organic compound, more accurately the methyl ester of acrylic acid. It is a colourless liquid with a characteristic acrid odor. It is mainly produced to make acrylate fiber, which is used to weave synthetic carpets. It is also a reagent in the synthesis of various pharmaceutical intermediates.
The standard industrial reaction for producing methyl acrylate is esterification with methanol under acid catalysis (sulfuric acid, p-toluene sulfonic acid, acidic ion exchangers.). The transesterification is facilitated because methanol and methyl acrylate form a low boiling azeotrope (b.p. 62-63 °C).
The patent literature describes a one-pot route involving vapor-phase oxidation of propene or 2-propenal with oxygen in the presence of methanol.
Methyl acrylate can be prepared by debromination of methyl 2,3-dibromopropanoate with zinc. Methyl acrylate is formed in good yield on pyrolysis of methyl lactate in the presence of ethenone (ketene). Methyl lactate is a renewable "green chemical". Another patent describes the dehydration of methyl lactate over zeolites.
The nickel tetracarbonyl-catalyzed hydrocarboxylation of acetylene with carbon monoxide in the presence of methanol also yields methyl acrylate. The reaction of methyl formate with acetylene in the presence of transition metal catalysts also leads to methyl acrylate. Both, the alcoholysis of propiolactone with methanol as well as the methanolysis of acrylonitrile via intermediately formed acrylamide sulfate are also proven but obsolete processes.
...
Wikipedia