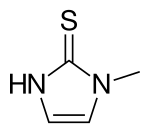
Methimazole
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Tapazole, Thiamazole, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682464 |
| Pregnancy category |
|
| Routes of administration |
by mouth |
| ATC code | H03BB02 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 93% |
| Protein binding | None |
| Metabolism | Liver |
| Biological half-life | 5-6 hours |
| Excretion | Kidney |
| Identifiers | |
|
|
| CAS Number |
60-56-0 |
| PubChem (CID) | 1349907 |
| IUPHAR/BPS | 6649 |
| DrugBank |
DB00763 |
| ChemSpider |
1131173 |
| UNII |
554Z48XN5E |
| KEGG |
D00401 |
| ChEBI |
CHEBI:50673 |
| ChEMBL |
CHEMBL1515 |
| ECHA InfoCard | 100.000.439 |
| Chemical and physical data | |
| Formula | C4H6N2S |
| Molar mass | 114.17 g/mol |
| 3D model (Jmol) | Interactive image |
| Melting point | 146 °C (295 °F) |
| Solubility in water | 275 mg/mL (20 °C) |
|
|
|
|
Methimazole (MMI), sold under the brand name Thiamazole among others, is an antithyroid drug, and part of the thioamide group. Like its counterpart propylthiouracil, a major side effect of treatment is agranulocytosis.
Methimazole is a drug used to treat hyperthyroidism such as in Graves' disease, a condition that occurs when the thyroid gland begins to produce an excess of thyroid hormone. The drug may also be taken before thyroid surgery to lower thyroid hormone levels and minimize the effects of thyroid manipulation. Additionally, methimazole is used in the veterinary setting to treat hyperthyroidism in cats.
It is important to monitor any symptoms of fever or sore throat while taking methimazole; this could indicate the development of agranulocytosis, an uncommon but severe side effect resulting from a drop in the white blood cell count (to be specific, neutropenia, a deficiency of neutrophils). A complete blood count (CBC) with differential is performed to confirm the suspicion, in which case the drug is discontinued. Administration of recombinant human granulocyte colony-stimulating factor (rhG-CSF) may increase recovery.
Other known side effects include:
Adverse effects may occur for individuals who:
Methimazole has been demonstrated to potently inhibit all CYP450 enzymes, meaning that the plasma concentration of all hepatically metabolized drugs will be significantly increased when taken with, or after, methimazole. This must be considered when starting a patient on methimazole, increasing their dose, or starting a methimazole patient on another drug. Many drugs will need their doses lowered and dosing-time intervals increased when co-administered with methimazole. For some hepatically metabolized drugs with very low therapeutic indexes, co-administration with methimazole may not be possible at all.
Methimazole inhibits the enzyme thyroperoxidase, which normally acts in thyroid hormone synthesis by oxidizing the anion iodide (I−) to iodine (I2), hypoiodous acid (HOI), enzyme linked hypoiodate (EOI) facilitating iodine's addition to tyrosine residues on the hormone precursor thyroglobulin, a necessary step in the synthesis of triiodothyronine (T3) and thyroxine (T4).
...
Wikipedia
