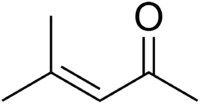
Mesityl oxide
 |
|
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
4-methylpent-3-en-2-one
|
|
| Other names
Mesityl oxide
Isobutenyl methyl ketone Methyl isobutenyl ketone Isopropylidene acetone |
|
| Identifiers | |
|
141-79-7 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
8526 |
| ECHA InfoCard | 100.005.002 |
| RTECS number | SB4200000 |
|
|
|
|
| Properties | |
| C6H10O | |
| Molar mass | 98.15 g·mol−1 |
| Appearance | Oily, colorless to light-yellow liquid |
| Odor | peppermint- or honey-like |
| Density | 0.858 g/cm3 |
| Melting point | −53 °C (−63 °F; 220 K) |
| Boiling point | 129.5 °C (265.1 °F; 402.6 K) |
| 3% (20°C) | |
| Solubility in other solvents | Soluble in most organic solvents |
| Vapor pressure | 9 mmHg (20°C) |
|
Refractive index (nD)
|
1.442 |
| Hazards | |
| Main hazards | flammable |
| R-phrases | R10 R20/21/22 |
| S-phrases | S25 |
| Flash point | 31 °C; 87 °F; 304 K |
| Explosive limits | 1.4%-7.2% |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
1120 mg/kg (rat, oral) 1000 mg/kg (rabbit, oral) 710 mg/kg (mouse, oral) |
|
LC50 (median concentration)
|
1000 mg/m3 (rat, 4 hr) 9000 mg/m3 (rat, 4 hr) 10,000 mg/m3 (mouse, 2 hr) 2000 mg/m3 (guinea pig, 7 hr) |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
TWA 25 ppm (100 mg/m3) |
|
REL (Recommended)
|
TWA 10 ppm (40 mg/m3) |
|
IDLH (Immediate danger)
|
1400 ppm |
| Related compounds | |
|
Related compounds
|
diacetone alcohol acetone, benzylideneacetone |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Mesityl oxide is a α,β-Unsaturated ketone with the formula CH3C(O)CH=C(CH3)2. This compound is a colorless, volatile liquid with a strong cat urine odor.
It is prepared by the aldol condensation of acetone to give diacetone alcohol, which readily dehydrates to give this compound.
Isophorone may be formed under the same conditions of mesityl oxide production, by a Michael addition. The yields of mesityl oxide and isophorone may vary according to reaction conditions during synthesis:
Mesityl oxide is used as a solvent and in the production of methyl isobutyl ketone by hydrogenation:
...
Wikipedia
